Abstract
Introduction
Discerning whether range of motion (ROM) is restricted by morphology or other pain sources is challenging in patients with femoroacetabular impingement syndrome (FAIS). Computed tomography (CT) motion simulation provides a hypothetical ROM based on morphology. This study aimed to explore associations between ROM measured using CT motion simulation and maximum passive ROM measured clinically using three dimensional (3D) motion analysis in patients with FAIS, prior to and post arthroscopic hip surgery.
Materials and methods
Eight males with FAIS (in total 12 hip joints) were included in this explorative feasibility study. Participants were examined using CT according to a low-dose protocol prior to and 7-months post arthroscopic surgery. Software was used to simulate at which ROM the impingement would occur. With the hip in 90 degrees’ flexion, maximum passive range of internal hip rotation, and maximum passive internal hip rotation coupled with adduction was examined clinically using 3D motion analysis pre- and postoperatively. Spearman rank correlation coefficients and linear regressions examined associations between methods.
Results
Preoperatively, the correlation between maximum internal hip rotation measured using CT motion simulation and 3D motion analysis was strong (r = 0.71, p = 0.009). Linear regressions demonstrated that maximal internal rotation measured using CT motion simulation was predominantly larger than when measured using 3D motion analysis. Postoperatively, and when maximum internal rotation was coupled with adduction, no correlations were found between the two methods.
Conclusions
The hypothetical morphology restricted ROM is larger than clinically assessed pain restricted ROM, both prior to and post hip arthroscopy. These findings suggest that ROM is restricted by pain rather than mechanical, morphology-based impingement in individuals with FAIS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Femoroacetabular impingement (FAI), is a condition where the bones of the hip joint do not have a matching shape. Cam impingement or deformity, refers to a cam effect initiated by a non-spherical femoral head rotating inside the acetabulum [1], while pincer impingement involves focal or general over coverage by the acetabulum of the femoral head [2]. During movement this mismatch may lead to joint friction and reduced range of motion (ROM) [1, 3, 4]. Depending on the severity of the morphologic mismatch and an individual’s activity the condition is associated with cartilage damage [5], eventually causing pain and femoroaceatabular impingement syndrome (FAIS) [1, 3]. It is important to remember that morphologic features and labral injuries are common also in asymptomatic individuals [6]. The specificity for radiographic imaging signs in predicting risk for developing FAIS is poor, since cam changes have been documented in 55% of asymptomatic athletes and 23% in an asymptomatic general population [6, 7].
The main symptoms of FAIS is motion-related or position-related pain in the hip or groin [8]. The onset of pain is usually gradual, without previous known trauma. In addition, pain may also be manifested in the back, buttock or thigh, and patients may also describe restricted ROM, stiffness, clicking, catching or locking sensations [8]. Since these symptoms are similar to those of several differential diagnoses, receiving the diagnosis ‘Femoroacetabular Impingement Syndrome’ often takes time, which potentially delays appropriate treatment. Today, FAIS is diagnosed based on medical history and findings from clinical examination and radiography [8]. Typically, the medical history reveals restricted ROM in deep end range hip flexion, combined with internal rotation and hip adduction [3, 8]. In clinical practice, ROM is routinely measured using goniometers, limiting measures to only include one plane at a time. Three-dimensional (3D) motion analysis is a method based on biomechanical models that use external skin markers to define a local coordinate system to each skeletal segment which enables calculations of joint kinematics in all three planes [9].
Computed tomography (CT) provides additional information, compared to radiography, including 3D information of the joint. In 2007, a clinical pilot study examined a CT based method (‘HipMotion’) for noninvasive 3D assessment of FAI [10]. HipMotion was found to be a reliable, accurate, and noninvasive clinical tool for assessment of FAI, and further, a means for planning of surgical interventions [10]. Advances in imaging techniques nowadays enable use of low-dose CT, with radiation levels comparable to radiography [11]. In 2015, a cadaveric study was performed to evaluate the ability of a simulation software to determine deformities limiting hip joint dynamics based on CT imaging [12]. The technique demonstrated potential to serve as a clinical diagnostic tool for patients presenting with FAI symptoms, as it provides a hypothetical ROM based on morphology. However, among patients with FAIS it remains unclear to which extent ROM is restricted due to morphology or by other pain sources. This feasibility study aimed to explore associations between ROM measured using CT motion simulation and maximum passive ROM measured clinically using 3D motion analysis in patients with FAIS, prior to and post arthroscopic hip surgery.
Materials and methods
The study was reported following the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) statement as a guideline [13]. The study was approved by the regional ethical review board in Stockholm, Sweden, (DNR 015/467–31) and Radiation Protection Committee (Strålskyddskommittén) at Karolinska University Hospital (DNR K1031-2015). Eight male study participants subject to hip arthroscopy were prospectively recruited from Capio Artro Clinic, Sophiahemmet, Stockholm, Sweden between September 2015 and August 2016. Inclusion criteria included a typical history of FAIS, radiographic findings of cam FAI with an alpha angle > 60 degrees, with or without signs of pincer, 15–40 years of age, symptom duration > 1 year, and being scheduled for arthroscopic hip surgery for FAIS (Table 1). Exclusion criteria included previous surgery of the hip, osteoarthritis (cartilage height < 2 mm on radiographic examination of the hip according to Tönnis et al. [14]), hip dysplasia (defined as Wiberg angle < 25 degrees), rheumatoid arthritis, neurological disease, Mb Perthes, and/or body mass index (BMI) > 30. All arthroscopic hip surgeries were performed at Capio Artro Clinic, Sophiahemmet, Stockholm, Sweden, by two senior consultant orthopedic surgeons.
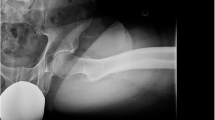
Hip joints of study participants were examined prior to and at median 7 months (range 4–11) following arthroscopic hip surgery using a Toshiba Dual-Energy CT at Karolinska University Hospital, Solna, Sweden, according to a low-dose protocol. Motion simulation was conducted using the simulation software, Articulis software (Clinical Graphics, Den Haag, The Netherlands). The software automatically converts the CT scans to 3D models of the femur and pelvis. The software identifies the impinging area by 0.1 mm and calculates the amount of bone necessary to resect to create a hip configuration without impingement (Fig. 1) [12]. Simulated values (measured in degrees) of maximum internal rotation with the hip in 90 degrees’ flexion, and maximum internal rotation plus adduction with the hip in 90 degrees’, respectively, were used in the analysis.
Computed tomography (CT) motion simulation using Articulis software (Clinical Graphics, Den Haag, The Netherlands). The software automatically converts CT scans to 3D models of the femur and pelvis. In this figure the hip joint is presented in an anterior–posterior projection with a the hip joint in neutral b the hip joint in 90 degrees’ flexion and 26 degrees’ internal rotation when the impingement occurs. The software identifies the impinging area by 0.1 mm
3D motion analysis was performed at the Motion Analysis laboratory at Karolinska University Hospital, Solna, Sweden, prior to and at median 7 months after surgery. Biomechanical measurements were obtained using an 8-camera system (Vicon Motion Systems Ltd, Oxford, UK). A conventional biomechanical model, the Plug-in-Gait model, including 18 reflective markers was used. Good intra-sessional repeatability has been reported using the Plug-in-Gait model [15], and for global kinematic data in healthy adults an inter-sessional standard error of 1.8 degrees [16]. Calculations of 3D motion analysis data were performed using MATLAB® software R2014a (The MathWorks, Inc., Natick, MA). Passive ROM of the hip joint was examined with the participant in a supine position, with reflective markers on, to record hip kinematics (i.e. maximum ROM of body segments) in three planes; frontal, sagittal and transversal. One examiner fixated the participant’s hip manually in 90 degrees’ flexion. A second examiner passively moved the joint towards maximum internal rotation. Next, with the hip still manually fixated in 90 degrees of flexion, maximum internal rotation plus adduction was evaluated. Study participants were asked to rate their perceived pain during the test of passive ROM using a visual analog scale (VAS). The VAS is a commonly used psychometric response scale for assessing pain, consisting of a 10 cm long line where the participant indicates how much pain he or she is experiencing [17]. It is measured in millimeters were 0 means “No pain at all” and 100 mm mean “Worst imaginable pain”.
Statistical analysis
Due to the nature of this explorative feasibility study, no sample size calculation was performed prior to the study. Statistical analyses were performed using IBM SPSS Statistics version 26 (Chicago, IL). A significance level was set at ɑ = 0.05. Mean, standard deviation, median, range, and percentage described variables. Spearman rank correlation coefficients examined the strength of the relationship between ROM measured using CT motion simulation and 3D motion analysis [18]. Correlation coefficients were interpreted according to Dancey and Reidy, where an r-value of ± 1 is considered a perfect correlation, ± 0.7 to ± 0.9 strong, ± 0.4 to ± 0.6 moderate, ± 0.1 to ± 0.3 weak, and 0 inferring no correlation [19]. In case of a significant correlation, the relationship between methods was explored using univariate linear regressions to provide an estimate of the amount the dependent variable (3D motion analysis) increase with one unit increase in the independent variable (CT motion simulation) [20]. Paired sample t-tests were used to evaluate pre- to postoperative changes in ROM on group level. Differences in absolute ROM, prior to and after surgery, are presented and illustrated graphically (Figs. 2 and 3). Change in VAS pain, prior to and after surgery, was evaluated using Wilcoxon Signed Rank test.
Maximum passive range of internal rotation with the hip in 90 degrees’ flexion measured using computed tomography (CT) motion simulation and three-dimensional (3D) motion analysis a prior to and b at mean 7 months after arthroscopic surgery. For participants with no orange bar, the CT simulation yielded 0 degrees of internal rotation with the hip in 90 degrees’ flexion
Maximum passive range of internal rotation and adduction with the hip in 90 degrees’ flexion measured using computed tomography (CT) motion simulation and three-dimensional (3D) motion analysis a prior to and b at mean 7 months after arthroscopic surgery. For participants with no orange bar, the CT simulation yielded 0 degrees of internal rotation with the hip in 90 degrees’ flexion and adduction
Results
Demographics of included study participants
Eight males with a mean age of 27.2 years (SD 7.3), mean height 176 cm (SD 7.7), and mean BMI 21.1 (SD 2.1), were included in the study. Four of the included participants had bilateral FAIS and thus, in total, measurements from 12 hip joints were examined and included in the analysis (Table 1). Surgical resection of cam impingement was performed in all 12 examined hip joints, and surgical resection of pincer impingement was performed in three hip joints (Table 2). No labrum sutures were performed.
Correlation between preoperative range of motion
Prior to surgery, the correlation between maximum internal rotation measured using CT motion simulation and 3D motion analysis was strong (Table 3). Linear regressions demonstrated that maximum internal rotation measured using CT motion simulation was predominantly larger than when measured using 3D motion analysis (Table 4, Figs. 2 and 3). On average, the difference between CT motion simulation and 3D motion analysis was 16 degrees (Table 4). No correlation between methods was found when preoperative maximum internal rotation coupled with adduction was examined (Table 3).
Correlation between postoperative range of motion
At a median duration of 7 months after arthroscopic hip surgery, no correlations were found between ROM measured using CT motion simulation and 3D motion analysis (Table 3).
Pre- to postoperative change in range of motion
After surgery, the majority of included participants displayed increased ROM of the hip based on CT motion simulation and passive ROM of the hip measured using 3D motion analysis (Figs. 2 and 3). On group level, irrespective of evaluation method and movements examined, no significant pre-to postoperative changes in hip joint ROM were found after surgery (Table 5).
Perceived pain
At the preoperative test session of clinically assessed ROM, study participants rated their perceived pain on the VAS to median 65 mm (range 20–93). After surgery, pain was significantly reduced to median VAS 28 mm (range 0–65) (p = 0.005).
Discussion
This study aimed at exploring associations between bone-on-bone morphology-based hip joint ROM and pain restricted ROM in individuals with FAIS prior to and following arthroscopic hip surgery. To this end, range of internal hip rotation, and internal hip rotation coupled with adduction, was assessed using CT motion simulation and clinically using 3D motion analysis. The results demonstrated a strong correlation between the methods prior to surgery when maximum internal rotation was assessed. Linear regressions further demonstrated that internal hip rotation measured using CT motion simulation was predominantly larger than clinically assessed pain restricted ROM. When maximum internal hip rotation was combined with hip adduction no correlations were found. After surgery, no linear relationship between methods was found, this with respect to both maximum internal rotation, as well as internal rotation coupled with adduction.
Partly corroborating findings of the present study, Bedi et al., compared clinician measured ROM and CT based computer model estimated ROM in 10 hip joints with FAI, pre and post arthroscopic osteoplasty [21]. Results demonstrated significant correlations between the clinical measurements and the ROM predicted by the model. In contrast to results of the present study, Bedi and colleagues demonstrated that both hip flexion and internal rotation ROM were improved postoperatively, according to both the clinical assessment and the computer model. The authors conclude that, in symptomatic patients, the magnitude of improvement in ROM is not predictable based on radiographic measures alone. However, CT based computer modeling can be utilized to localize regions of anticipated mechanical impingement [21].
Prior to surgery, CT simulated internal hip rotation was predominantly larger than clinically assessed passive internal hip rotation measured using 3D motion analysis. On average, CT motion simulation yielded a ROM 16 degrees larger than 3D motion analysis. The simulated range of motion based on CT scans describe a theoretically possible ROM, allowed by the patient’s bone conformation, while for clinically assessed passive ROM, movement is affected by pain, discomfort, and possibly the labrum [22]. CT motion simulation may oversimplify hip contact mechanics, since these models include bone-to-bone contact but remove all soft tissue, including the cartilage and labrum [22]. Findings of a recent study, examining the effect of inclusion of the acetabular labrum on maximum ROM during simulation of the flexion–adduction–internal rotation impingement, demonstrated reduced internal rotation ROM by close to 20° and increased variability in the location of contact relative to the acetabular rim [22]. Many patients with FAIS have concomitant hip and groin pain originating from other sources, i.e. adductor-related pathology and soft tissues [23, 24] (i.e. muscles, tendons, ligament, and joint capsule), which may be contributing limiting factors. In addition, cartilage injuries and degenerative changes on joint surfaces commonly seen with FAIS also contribute to pain and limitations in function [25]. Consequently, deciphering the root of pain is difficult in FAIS.
No correlation between CT motion simulation and 3D motion analysis was found when internal rotation plus adduction was evaluated, although measurements were more aligned between methods at the preoperative assessment than at the postoperative assessment (preoperative: r = 0.52, p = 0.08, postoperative: r = − 18, p = 0.56). Evaluation of hip joint range of motion in all three planes simultaneously is complex and difficult to measure clinically. In combination with additional stress on soft tissues, this could be a contributing factor to the statistically non-significant correlations found when adduction was added as compared to just flexion and internal rotation. This may further be reflected in the larger discrepancy seen postoperatively between the simulated and clinically assessed ROM (Fig. 3) where response to surgery is yet an influential factor on clinically assessed ROM. The comprehensive movement performed; hip flexion, adduction, and internal rotation (FADIR) is a commonly used examination test for FAI [26]. The test is considered positive if pain in end range movement occurs. However, the FADIR test has been reported to render a large number of false positive tests among adolescent ice hockey players without diagnosed hip disorder, and thus be inadequate for screening cam and pincer morphology [27]. Palmer et al. evaluated outcomes 8 months after arthroscopic hip surgery for FAIS and compared to physiotherapy and activity modification in a pragmatic randomized controlled study [28]. The authors found that patients treated with arthroscopic surgery displayed increased hip flexion postoperatively, however, remained fairly unchanged in hip adduction and internal rotation. At the 8-month follow-up after surgery, 63% of patients had a positive FADIR test [28]. In the light of previous research, where a false positive FADIR test is common in athletes without hip disorder, as well as a common finding in individuals with FAIS after surgery, the absent correlation between CT motion simulation and clinically assessed FADIR should not come as a surprise, neither preoperatively nor postoperatively.
After surgery, the majority of included patients displayed increased ROM of the hip based on CT motion simulation and passive ROM of the hip measured using 3D motion analysis. However, a few individuals demonstrated unchanged ROM after surgery, both according to CT motion simulation, and 3D motion analysis. Irrespective of evaluation method used, or movement combinations evaluated, no significant increase in range of motion on group level was observed postoperatively. After surgery, the number of individuals with a simulated (hypothetical) full range of internal rotation (i.e. 30 degrees) was greater when measured with hip flexion only, and not coupled with adduction. This result may reflect that it is not always technically possible to resect the entire bony prominence of the femoral head circumference to create a hip configuration without impingement using arthroscopy.
In the present study, ROM was re-evaluated at median 7 months after surgery. The timing of the postoperative evaluation may have impacted the results as pain was reduced, yet still present during test of passive maximal ROM. Preoperatively, study participants rated their perceived pain to be median VAS 65 (range 20–93). After surgery, pain was significantly reduced, although still present (median VAS 28, range 0–65). An alternative approach, which possibly could have rendered a larger ROM during the clinical assessment, and perhaps a stronger correlation between methods, would have been to inject the hip joint with local anesthesia. In line with our results, a recent cross sectional study evaluating objective hip-related function in patients following hip arthroscopy reported less hip mobility 6 to 10 months after surgery as compared to controls [29]. Previous studies evaluating patient-reported outcomes, including pain measurements, following hip arthroscopy report improvements at one and two years after surgery [30,31,32,33,34], indicating postoperative improvements beyond the time span evaluated in the present study. Furthermore, a recent meta-analysis evaluating return to sports following hip arthroscopy (for FAIS) concluded that the observed mean duration for return to play was 7.4 months [35]. Consequently, timing of the follow-up in the present study may be considered too early after surgery in a pain-perspective, yet reasonable in a functional mobility perspective.
This study holds several limitations. The study is based on a small number of observations, consequently, the risk for type two errors is evident, and the external validity as well as generalizability of the results are limited. The time period to follow-up may be considered too short as previously discussed, as further improvements during the first year postoperatively may be expected [30, 31]. The present study used 3D motion analysis to quantify the clinically assessed ROM, a method associated with limitations of its own. The biomechanical model Plug-in-Gait was used, which relies on anthropometric based regression equations to estimate the hip joint center [36]. The calculation is based on the width of the pelvis (to define the medial–lateral position of the hip joint center), and the distance between the anterior superior iliac and the greater trochanter [36]. Difficulties may occur when reflective markers cannot be placed directly on to the anterior superior iliac due to soft tissue. An alternative approach would have been to use a functional calibration method, where the hip joint center can be estimated from the movement of the thigh with respect to the pelvis during calibration trials [37]. However, in the present study, all included study participants had a BMI below 25 and no excessive soft tissue around the pelvis, hip or thigh. The movements examined were carried out passively by an examiner at slow speed, consequently, movement artefacts of the reflective markers due to soft tissue was not a problem. It was one and the same investigator placing the reflective markers for all 3D motion analyses, and the errors related to the biomechanical model did not change between pre- and post-evaluation, and should therefore, not have a huge impact on the results. Nevertheless, the estimated hip joint center may affect the measured ROM.
Conclusion
Findings of this study suggest that ROM is restricted by pain to a greater extent than mechanical, bone-on-bone, morphology-based impingement in individuals with FAIS both pre- and postoperatively. Prior to arthroscopic hip surgery, the correlation between clinically assessed maximum internal hip rotation and ROM measured using CT motion simulation is strong. CT motion simulation, exclusive labrum, cartilage and soft tissues, indicates a greater theoretically possible ROM, however, postoperatively no statistically significant correlation to clinically assessed ROM was found, indicating less predictable postoperative results on pain and function.
Availability of data and material
The datasets that are used and analyzed for the present study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA (2003) Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 417:112–120. https://doi.org/10.1097/01.blo.0000096804.78689.c2
Tannast M, Siebenrock KA, Anderson SE (2007) Femoroacetabular impingement: radiographic diagnosis–what the radiologist should know. AJR Am J Roentgenol 188(6):1540–1552. https://doi.org/10.2214/ajr.06.0921
Beck M, Kalhor M, Leunig M, Ganz R (2005) Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br 87(7):1012–1018. https://doi.org/10.1302/0301-620x.87b7.15203
Byrd JW (2010) Femoroacetabular impingement in athletes, part 1: cause and assessment. Sports Health 2(4):321–333. https://doi.org/10.1177/1941738110368392
Valera M, Ibáñez N, Sancho R, Llauger J, Gich I (2018) Acetabular overcoverage in the horizontal plane: an underdiagnosed trigger of early hip arthritis. A CT scan study in young adults. Arch Orthop Trauma Surg 138(1):73–82. https://doi.org/10.1007/s00402-017-2811-y
Frank JM, Harris JD, Erickson BJ, Slikker W 3rd, Bush-Joseph CA, Salata MJ, Nho SJ (2015) Prevalence of femoroacetabular impingement imaging findings in asymptomatic volunteers: a systematic review. Arthroscopy 6:1199–1204. https://doi.org/10.1016/j.arthro.2014.11.042
Hack K, Di Primio G, Rakhra K, Beaule PE (2010) Prevalence of cam-type femoroacetabular impingement morphology in asymptomatic volunteers. J Bone Joint Surg Am 92(14):2436–2444. https://doi.org/10.2106/jbjs.j.01280
Griffin DR, Dickenson EJ, O’Donnell J, Agricola R, Awan T, Beck M, Clohisy JC, Dijkstra HP, Falvey E, Gimpel M, Hinman RS, Holmich P, Kassarjian A, Martin HD, Martin R, Mather RC, Philippon MJ, Reiman MP, Takla A, Thorborg K, Walker S, Weir A, Bennell KL (2016) The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med 50(19):1169–1176. https://doi.org/10.1136/bjsports-2016-096743
Wu G, Cavanagh PR (1995) ISB recommendations for standardization in the reporting of kinematic data. J Biomech 28(10):1257–1261. https://doi.org/10.1016/0021-9290(95)00017-c
Tannast M, Kubiak-Langer M, Langlotz F, Puls M, Murphy SB, Siebenrock KA (2007) Noninvasive three-dimensional assessment of femoroacetabular impingement. J Orthop Res 25(1):122–131. https://doi.org/10.1002/jor.20309
Becce F, Ben Salah Y, Verdun FR, Vande Berg BC, Lecouvet FE, Meuli R, Omoumi P (2013) Computed tomography of the cervical spine: comparison of image quality between a standard-dose and a low-dose protocol using filtered back-projection and iterative reconstruction. Skeletal Radiol 42(7):937–945. https://doi.org/10.1007/s00256-013-1576-9
Roling MA, Visser MI, Oei EH, Pilot P, Kleinrensink GJ, Bloem RM (2015) A quantitative non-invasive assessment of femoroacetabular impingement with CT-based dynamic simulation–cadaveric validation study. BMC Musculoskelet Disord 16:50. https://doi.org/10.1186/s12891-015-0504-7
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2014) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 12(12):1495–1499. https://doi.org/10.1016/j.ijsu.2014.07.013
Tonnis D, Heinecke A (1999) Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am 81(12):1747–1770. https://doi.org/10.2106/00004623-199912000-00014
Ferrari A, Benedetti MG, Pavan E, Frigo C, Bettinelli D, Rabuffetti M, Crenna P, Leardini A (2008) Quantitative comparison of five current protocols in gait analysis. Gait Posture 28(2):207–216. https://doi.org/10.1016/j.gaitpost.2007.11.009
Eve L, Mc Nee A, Shortland A (2006) Extrinsic and intrinsic variation in kinematic data from the gait of healthy adult subjects. Gait Posture 24(Supplement 2):S56–S57. https://doi.org/10.1016/j.gaitpost.2006.11.041
Kahl C, Cleland J (2005) Visual analogue scale, numeric pain rating scale and the McGill pain Questionnaire: an overview of psychometric properties. Phys Ther Rev 10(2):123–128. https://doi.org/10.1179/108331905x55776
Liu J, Tang W, Chen G, Lu Y, Feng C, Tu XM (2016) Correlation and agreement: overview and clarification of competing concepts and measures. Shanghai Arch Psychiatry 28(2):115–120. https://doi.org/10.11919/j.issn.1002-0829.216045
Dancey CP, Reidy J (2007) Statistics without maths for psychology: using SPSS for Windows. Pearson/Prentice Hall, Harlow, England
Campbell MJ (2007) Medical statistics: a textbook for the health sciences. 4. ed. edn. Chichester, Wiley, Chichester
Bedi A, Dolan M, Hetsroni I, Magennis E, Lipman J, Buly R, Kelly BT (2011) Surgical treatment of femoroacetabular impingement improves hip kinematics: a computer-assisted model. Am J Sports Med 39(Suppl):43s–49s. https://doi.org/10.1177/0363546511414635
Atkins PR, Hananouchi T, Anderson AE, Aoki SK (2020) Inclusion of the acetabular labrum reduces simulated range of motion of the hip compared with bone contact models. Arthrosc Sports Med Rehabil 2(6):e779–e787. https://doi.org/10.1016/j.asmr.2020.07.014
de Sa D, Holmich P, Phillips M, Heaven S, Simunovic N, Philippon MJ, Ayeni OR (2016) Athletic groin pain: a systematic review of surgical diagnoses, investigations and treatment. Br J Sports Med 50(19):1181–1186. https://doi.org/10.1136/bjsports-2015-095137
Sansone M, Ahlden M, Jonasson P, Thomee R, Falk A, Sward L, Karlsson J (2014) Can hip impingement be mistaken for tendon pain in the groin? A long-term follow-up of tenotomy for groin pain in athletes. Knee Surg Sports Traumatol Arthrosc 22(4):786–792. https://doi.org/10.1007/s00167-013-2738-y
King MG, Lawrenson PR, Semciw AI, Middleton KJ, Crossley KM (2018) Lower limb biomechanics in femoroacetabular impingement syndrome: a systematic review and meta-analysis. Br J Sports Med 52(9):566–580. https://doi.org/10.1136/bjsports-2017-097839
Reiman MP, Thorborg K (2015) Femoroacetabular impingement surgery: are we moving too fast and too far beyond the evidence? Br J Sports Med 49(12):782–784. https://doi.org/10.1136/bjsports-2014-093821
Casartelli NC, Brunner R, Maffiuletti NA, Bizzini M, Leunig M, Pfirrmann CW, Sutter R (2018) The FADIR test accuracy for screening cam and pincer morphology in youth ice hockey players. J Sci Med Sport 21(2):134–138. https://doi.org/10.1016/j.jsams.2017.06.011
Palmer AJR, Ayyar Gupta V, Fernquest S, Rombach I, Dutton SJ, Mansour R, Wood S, Khanduja V, Pollard TCB, McCaskie AW, Barker KL, Andrade T, Carr AJ, Beard DJ, Glyn-Jones S, Group FS (2019) Arthroscopic hip surgery compared with physiotherapy and activity modification for the treatment of symptomatic femoroacetabular impingement: multicentre randomised controlled trial. BMJ 364:l185. https://doi.org/10.1136/bmj.l185
Worner T, Nilsson J, Thorborg K, Granlund V, Stalman A, Eek F (2019) Hip function 6 to 10 months after arthroscopic surgery: a cross-sectional comparison of subjective and objective hip function, including performance-based measures, in patients versus controls. Orthop J Sports Med 7(6):2325967119844821. https://doi.org/10.1177/2325967119844821
Sansone M, Ahlden M, Jonasson P, Thomee C, Sward L, Ohlin A, Baranto A, Karlsson J, Thomee R (2017) Outcome after hip arthroscopy for femoroacetabular impingement in 289 patients with minimum 2-year follow-up. Scand J Med Sci Sports 27(2):230–235. https://doi.org/10.1111/sms.12641
Minkara AA, Westermann RW, Rosneck J, Lynch TS (2019) Systematic review and meta-analysis of outcomes after hip arthroscopy in femoroacetabular impingement. Am J Sports Med 47(2):488–500. https://doi.org/10.1177/0363546517749475
Larson CM, Giveans MR, Samuelson KM, Stone RM, Bedi A (2014) Arthroscopic hip revision surgery for residual femoroacetabular impingement (FAI): surgical outcomes compared with a matched cohort after primary arthroscopic FAI correction. Am J Sports Med 42(8):1785–1790. https://doi.org/10.1177/0363546514534181
Kierkegaard S, Dalgas U, Lund B, Lipperts M, Søballe K, Mechlenburg I (2020) Despite patient-reported outcomes improve, patients with femoroacetabular impingement syndrome do not increase their objectively measured sport and physical activity level 1 year after hip arthroscopic surgery. Results from the HAFAI cohort. Knee Surg Sports Traumatol Arthrosc 28(5):1639–1647. https://doi.org/10.1007/s00167-019-05503-5
Kierkegaard S, Langeskov-Christensen M, Lund B, Naal FD, Mechlenburg I, Dalgas U, Casartelli NC (2017) Pain, activities of daily living and sport function at different time points after hip arthroscopy in patients with femoroacetabular impingement: a systematic review with meta-analysis. Br J Sports Med 51(7):572–579. https://doi.org/10.1136/bjsports-2016-096618
O’Connor M, Minkara AA, Westermann RW, Rosneck J, Lynch TS (2018) Return to play after hip arthroscopy: a systematic review and meta-analysis. Am J Sports Med 46(11):2780–2788. https://doi.org/10.1177/0363546518759731
Davis R, Ounpuu S, Tybursk D, Gage J (1991) A gait analysis data collection and reduction technique. Hum Mov Sci 10(5):575
Sangeux M, Pillet H, Skalli W (2014) Which method of hip joint centre localisation should be used in gait analysis? Gait Posture 40(1):20–25. https://doi.org/10.1016/j.gaitpost.2014.01.024
Konan S, Rayan F, Meermans G, Witt J, Haddad FS (2011) Validation of the classification system for acetabular chondral lesions identified at arthroscopy in patients with femoroacetabular impingement. J Bone Joint Surg Br 93(3):332–336. https://doi.org/10.1302/0301-620x.93b3.25322
Acknowledgements
The authors would like to acknowledge Professor Lanie Gutierrez-Farewik and data technician Mikael Reimeringer for assisting in data processing.
Funding
Open access funding provided by Karolinska Institute. No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors have no financial or proprietary interests in any material discussed in this article.
Ethics approval, consent, and data
All procedures performed in the present study were in accordance with the 1964 Helsinki declaration, the ethical standards of the regional ethical review board in Stockholm, Sweden, (DNR 015/467–31), and the Radiation Protection Committee (Strålskyddskommitten) at Karolinska University Hospital (DNR K1031-2015).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lars Weidenhielm deceased.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naili, J.E., Stålman, A., Valentin, A. et al. Hip joint range of motion is restricted by pain rather than mechanical impingement in individuals with femoroacetabular impingement syndrome. Arch Orthop Trauma Surg 142, 1985–1994 (2022). https://doi.org/10.1007/s00402-021-04185-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-021-04185-4