Abstract
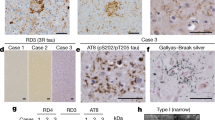
Dominantly inherited mutation D395G in the gene encoding valosin-containing protein causes vacuolar tauopathy, a type of behavioural-variant frontotemporal dementia, with marked vacuolation and abundant filamentous tau inclusions made of all six brain isoforms. Here we report that tau inclusions were concentrated in layers II/III of the frontotemporal cortex in a case of vacuolar tauopathy. By electron cryomicroscopy, tau filaments had the chronic traumatic encephalopathy (CTE) fold. Tau inclusions of vacuolar tauopathy share this cortical location and the tau fold with CTE, subacute sclerosing panencephalitis and amyotrophic lateral sclerosis/parkinsonism-dementia complex, which are believed to be environmentally induced. Vacuolar tauopathy is the first inherited disease with the CTE tau fold.







Similar content being viewed by others
Data availability
Cryo-EM maps have been deposited in the Electron Microscopy Data Bank (EMDB) with accession numbers: EMD-19926; EMD-19927; EMD-19928. Corresponding refined atomic models have been deposited in the Protein Data Bank (PDB) under the following accession numbers: 9ERM, 9ERN, 9ERO. Please address requests for materials to the corresponding authors.
References
Bodnar NO, Rapoport TA (2017) Molecular mechanism of substrate processing by the Cdc48 ATPase complex. Cell 169:722–725
Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kaprai GI et al (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D 66:12–21
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766
Croll TI (2018) ISOLDE: a physically realistic environment for model building into low-resolution elerctron density map. Acta Crystallogr D 74:519–530
Darwich NF, Phan JM, Kim E, Suh ER, Papatriantafyllou JD, Changolkar L et al (2020) Autosomal dominant VCP hypomorph mutation impairs disaggregation of PHF-tau. Science 370:eaay8826
Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D 66:486–501
Falcon B, Zivanov J, Zhang W, Murzin AG, Garringer HJ, Vidal R et al (2019) Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 568:420–423
Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ et al (2017) Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547:185–190
Goedert M, Spillantini MG, Cairns NJ, Crowther RA (1992) Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms. Neuron 8:159–168
Goedert M, Crowther RA, Scheres SHW, Spillantini MG (2024) Tau and neurodegeneration. Cytoskeleton 81:95–102
Guo H, Franken E, Deng Y, Benlekbir S, Lezcano GS, Janssen B et al (2020) Electron-event representation data enable efficient cryo-EM file storage with full preservation of spatial and temporal resolution. IUCrJ 7:860–869
Hasegawa M, Watanabe S, Kondo H, Akiyama H, Mann DMA, Saito Y et al (2014) 3R and 4R tau isoforms in paired helical filaments in Alzheimer’s disease. Acta Neuropathol 127:303–305
He S, Scheres SHW (2017) Helical reconstruction in RELION. J Struct Biol 193:163–176
Hof PR, Bouras C, Buée L, Delacourte A, Perl DP, Morrison JH (1992) Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer’s disease cases. Acta Neuropathol 85:23–30
Hof PR, Charpiot A, Delacourte A, Buée L, Purohit D, Perl DP et al (1992) Distribution of neurofibrillary tangles and senile plaques in the cerebral cortex in postencephalitic parkinsonism. Neurosci Lett 139:10–14
Kametani F, Yoshida M, Matsubara T, Murayama S, Saito Y, Kawakami I et al (2020) Comparison of common and disease-specific post-translational modifictions of pathological tau associated with a wide range of tauopathies. Front Neurosci 14:581936
Kawakatsu S, Kobayashi R, Hayashi H, Morioka D, Utsunomiya A, Kabasawa T et al (2021) Clinicopathological heterogeneity of Alzheimer’s disease with pure Alzheimer’s disease pathology: cases associated with dementia with Lewy bodies, very early-onset dementia, and primary progressive aphasia. Neuropathology 41:427–449
Keren-Shaul H, Spinrad A, Weinmer A, Matcovitch-Natan O, Dvir-Szrenfeld R, Ulland TK et al (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169:1276–1290
Kimanius D, Dong L, Sharov G, Nakane T, Scheres SHW (2021) New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem J 478:4169–4185
Kobayashi R, Naruse H, Kawakatsu S, Iseki C, Suzuki Y, Koyama S et al (2022) Valosin-containing protein Asp395Gly mutation in a patient with frontotemporal dementia: a case report. BMC Neurol 22:406
Kovacs GG, Ghetti B, Goedert M (2022) Classification of diseases with accumulation of tau protein. Neuropathol Appl Neurobiol 48:e12792
Lövestam S, Koh FA, van Knippenberg B, Kotecha A, Murzin AG, Goedert M et al (2022) Assembly of recombinant tau into filaments identical to those of Alzheimer’s disease and chronic traumatic encephalopathy. Elife 11:e76494
Lövestam S, Li D, Wagstaff JL, Kotecha A, Kimanius D, McLaughlin SH et al (2024) Disease-specific tau filaments assemble via polymorphic intermediates. Nature 625:119–125
Mimuro M, Yoshida S, Kuzuhara S, Kokubo Y (2018) Amyotrophic lateral sclerosis and parkinsonism-dementia complex of the Hohara focus of the Kii peninsula: a multiple proteinopathy? Neuropathology 38:98–107
Miyahara H, Akagi A, Riku Y, Sone J, Otsuka Y, Sakai M et al (2022) Independent distribution between tauopathy secondary to subacute sclerosing panencephalitis and measles virus. Brain Pathol 32:e13069
Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D 53:240–255
Murshudov GN, Skubák P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA et al (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D 67:255–267
Pearson RCA, Esiri MM, Hiorns RW, Wilcock GK, Powell TPS (1985) Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA 82:4531–4534
Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI et al (2011) Chimera X: structure visualization for researchers, editors and developers. Protein Sci 30:70–82
Pfeffer G, Lee G, Pontifex CS, Fanganiello RD, Peck A, Weihl CC et al (2022) Multisystem proteinopathy due to VCP mutations: a review of clinical heterogeneity and genetic diagnosis. Genes 13:963
Pollanen MS, Onzivua S, McKeever PM, Robertson J, Mackenzie IR, Kovacs GG et al (2023) The spectrum of disease and tau pathology of nodding syndrome in Uganda. Brain 146:954–967
Qi C, Hasegawa M, Takao M, Sakai M, Sasaki M, Mizutani M et al (2023) Identical tau filaments in subacute sclerosing panencephalitis and chronic traumatic encephalopathy. Acta Neuropathol Commun 11:74
Qi C, Verheijen BM, Kokubo Y, Shi Y, Tetter S, Murzin AG et al (2023) Tau filaments from amyotrophic lateral sclerosis/parkinsonism-dementia complex adopt the CTE fold. Proc Natl Acad Sci USA 120:e2306767120
Qi C, Lövestam S, Murzin AG, Peak-Chew S, Franco C, Bogdani M et al (2024) Tau filaments with the Alzheimer fold in cases with MAPT mutations V337M and R406W. BioRxiv. https://doi.org/10.1101/2024.04.29.591661
Ramos AM, Koros C, Dokuru DR, Van Berlo V, Kroupis C, Wojta K et al (2019) Frontotemporal dementia spectrum: first genetic screen in a Greek cohort. Neurobiol Aging 224:e1–e8
Rohou A, Grigorieff N (2015) CTFFIND4: fast and accurate defocus estimation from electron micrographs. J Struct Biol 192:216–221
Scheres SHW (2020) Amyloid structure determination in RELION-3.1. Acta Cryst D 76:94–101
Scheres SHW, Chen S (2012) Prevention of overfitting in cryo-EM structure determination. Nature Meth 9:8453–8454
Scheres SHW, Ryskeldi-Falcon B, Goedert M (2023) Molecular pathology of neurodegenerative diseases by cryo-EM of amyloids. Nature 621:701–710
Schmidt ML, Zhukareva V, Newell KL, Lee VMY, Trojanowski JQ (2001) Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer’s disease. Acta Neuropathol 101:5128–5524
Schweighauser M, Garringer HJ, Klingstedt T, Nilsson KPR, Masami-Suzukake M, Murrell JR et al (2023) Mutation ΔK281 in MAPT causes Pick’s disease. Acta Neuropathol 146:211–226
Shi Y, Murzin AG, Falcon B, Epstein A, Machin J, Tempest P et al (2021) Cryo-EM structures of tau filaments from Alzheimer’s disease with PET ligand APN-1607. Acta Neuropathol 141:697–708
Shi Y, Zhang W, Yang Y, Murzin AG, Falcon B, Kotecha A et al (2021) Structure-based classification of tauopathies. Nature 598:359–363
Shimozawa A, Ono M, Takahara D, Tarutani A, Imura S, Masuda-Suzukake M et al (2017) Propagation of pathological α-synuclein in marmoset brain. Acta Neuropathol Commun 5:12
Tarutani A, Arai T, Murayama S, Hisanaga SI, Hasegawa M (2018) Potent prion-like behaviors of pathogenic α–synuclein and evaluation of inactivation methods. Acta Neuropathol Commun 6:29
The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC
Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D et al (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 36:377–381
Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot O et al (2020) Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. Cell 183:1699–1713
Yamashita K, Palmer CM, Burnley T, Murshudov GN (2021) Cryo-EM single particle structure refinement and map calculation using Servalcat. Acta Crystallogr D 77:1282–1291
Zhu J, Pittman S, Dhavale D, French R, Patterson JN, Kaleelurrrahuman MS et al (2022) VCP suppresses proteopathic seeding in neurons. Mol Neurodegen 17:30
Zivanov J, Nakane T, Forsberg BO, Kimanius D, Hagen WJ, Lindahl E et al (2018) New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7:e42166
Zivanov J, Otón J, Ke Z, von Kügelen E, Pyle E, Qu K et al (2022) A Bayesian approach to single-particle electron-tomography in RELION-4.0. Elife 11:e83724
Acknowledgements
This work was supported by the Electron Microscopy Facility of the MRC Laboratory of Molecular Biology. We thank Jake Grimmett, Toby Darling and Ivan Clayson for help with high-performance computing, and Takumi Kitaoka and Mitsuru Futakuchi (Yamagata University School of Medicine) for help with neuropathology. We also thank Hiroya Naruse and Tatsushi Toda (University of Tokyo) for genomic analysis. For open access, the MRC Laboratory of Molecular Biology has applied a CC BY public copyright licence to any Author-Accepted Manuscript version arising.
Funding
This work was supported by the UK Medical Research Council (MC_UP_A025-1013 to S.H.W.S. and MC_U105184291 to M.G.), the Japanese Society for the Promotion of Science (JSPS KAKENHI and JP20K07922, to R.K. and S.K.) and the Japanese Ministry of Health, Labour and Welfare (JPMH20GB1002 and JPMH23GB1003, to R.K. and S.K.).
Author information
Authors and Affiliations
Contributions
RK and SK identified the patient and performed genetic analysis and neuropathology; MH prepared filaments and performed immunoblots; CQ performed cryo-EM data acquisition and structure determination; SHWS, MG and MH supervised the project and all authors contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
Studies carried out at Yamagata University were approved through the Institution’s ethical review process.
Informed consent
Informed consent was obtained from the patient’s next of kin. This study was approved by the Cambridgeshire 2 Research Ethics Committee (09/H0308/163).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
401_2024_2741_MOESM1_ESM.tif
Supplementary file1 (TIF 23627 KB) Fourier shell correlation (FSC) curves. FSC curves of cryo-EM maps (left panel) and model to map validation (right panel). a, CTE Type I tau filament from VT; CTE Type II tau filament from VT; CTE Type III tau filament from VT
401_2024_2741_MOESM2_ESM.tif
Supplementary file2 (TIF 9588 KB) Double-labelling immunofluorescence using anti-tau and anti-glial fibrillary acid protein antibodies. Sections of frontal cortex from the individual with mutation D395G in VCP were labelled with: a, anti-tau antibody AT8 (green) and anti-glial fibrillary acidic protein antibody (red); b, anti-tau antibody pS396 (red) and anti-glial fibrillary acidic protein antibody (green). DAPI nuclear staining is in blue. There was no evidence for the co-localisation of AT8, pS396 and glial fibrillary acidic protein. Scale bar, 50 m
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, C., Kobayashi, R., Kawakatsu, S. et al. Tau filaments with the chronic traumatic encephalopathy fold in a case of vacuolar tauopathy with VCP mutation D395G. Acta Neuropathol 147, 86 (2024). https://doi.org/10.1007/s00401-024-02741-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00401-024-02741-x