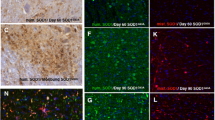
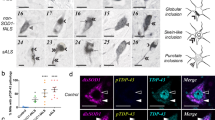
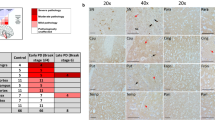
Abstract
Neuronal loss in numerous neurodegenerative disorders has been linked to protein aggregation and oxidative stress. Emerging data regarding overlapping proteinopathy in traditionally distinct neurodegenerative diseases suggest that disease-modifying treatments targeting these pathological features may exhibit efficacy across multiple disorders. Here, we describe proteinopathy distinct from classic synucleinopathy, predominantly comprised of the anti-oxidant enzyme superoxide dismutase-1 (SOD1), in the Parkinson’s disease brain. Significant expression of this pathology closely reflected the regional pattern of neuronal loss. The protein composition and non-amyloid macrostructure of these novel aggregates closely resembles that of neurotoxic SOD1 deposits in SOD1-associated familial amyotrophic lateral sclerosis (fALS). Consistent with the hypothesis that deposition of protein aggregates in neurodegenerative disorders reflects upstream dysfunction, we demonstrated that SOD1 in the Parkinson’s disease brain exhibits evidence of misfolding and metal deficiency, similar to that seen in mutant SOD1 in fALS. Our data suggest common mechanisms of toxic SOD1 aggregation in both disorders and a potential role for SOD1 dysfunction in neuronal loss in the Parkinson’s disease brain. This shared restricted proteinopathy highlights the potential translation of therapeutic approaches targeting SOD1 toxicity, already in clinical trials for ALS, into disease-modifying treatments for Parkinson’s disease.






Similar content being viewed by others
References
Ajroud-Driss S, Siddique T (2015) Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochem Biophys Acta 1852:679–684. doi:10.1016/j.bbadis.2014.08.010
Ayers JI, Xu G, Pletnikova O, Troncoso JC, Hart PJ, Borchelt DR (2014) Conformational specificity of the C4F6 SOD1 antibody; low frequency of reactivity in sporadic ALS cases. Acta Neuropathol Commun 2:55. doi:10.1186/2051-5960-2-55
Banci L, Bertini I, Boca M, Calderone V, Cantini F, Girotto S, Vieru M (2009) Structural and dynamic aspects related to oligomerization of apo SOD1 and its mutants. Proc Natl Acad Sci USA 106:6980–6985. doi:10.1073/pnas.0809845106
Bandmann O, Davis MB, Marsden CD, Harding AE (1995) Sequence of the superoxide-dismutase 1 (SOD1) gene in familial Parkinson’s disease. J Neurol Neurosurg Psychiatry 59:90–91. doi:10.1136/jnnp.59.1.90
Bartnikas TB, Gitlin JD (2003) Mechanisms of biosynthesis of mammalian copper/zinc superoxide dismutase. J Biol Chem 278:33602–33608. doi:10.1074/jbc.M305435200
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR (2015) Oxidative stress and Parkinson’s disease. Front Neuroanat 9:91. doi:10.3389/fnana.2015.00091
Braak H, Del Tredici K, Rub U, de Vos RAI, Steur E, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. doi:10.1016/s0197-4580(02)00065-9
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134. doi:10.1007/s00441-004-0956-9
Breydo L, Wu JW, Uversky VN (2012) Alpha-synuclein misfolding and Parkinson’s disease. Biochem Biophys Acta 1822:261–285. doi:10.1016/j.bbadis.2011.10.002
Brotherton TE, Li Y, Cooper D, Gearing M, Julien JP, Rothstein JD, Boylan K, Glass JD (2012) Localization of a toxic form of superoxide dismutase 1 protein to pathologically affected tissues in familial ALS. Proc Natl Acad Sci USA 109:5505–5510. doi:10.1073/pnas.1115009109
Burrow JN, Blumbergs PC (1992) Substantia nigra degeneration in motor neurone disease: a quantitative study. Aust N Z J Med 22:469–472
Chevreux S, Roudeau S, Fraysse A, Carmona A, Deves G, Solari PL, Mounicou S, Lobinski R, Ortega R (2009) Multimodal analysis of metals in copper-zinc superoxide dismutase isoforms separated on electrophoresis gels. Biochimie 91:1324–1327. doi:10.1016/j.biochi.2009.05.016
Choi J, Rees HD, Weintraub ST, Levey AI, Chin LS, Li L (2005) Oxidative modifications and aggregation of Cu, Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J Biol Chem 280:11648–11655. doi:10.1074/jbc.M414327200
Ciryam P, Kundra R, Morimoto RI, Dobson CM, Vendruscolo M (2015) Supersaturation is a major driving force for protein aggregation in neurodegenerative diseases. Trends Pharmacol Sci 36:72–77. doi:10.1016/j.tips.2014.12.004
Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM (2010) Synergistic interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. JNeurosci 30:7281–7289. doi:10.1523/JNEUROSCI.0490-10.2010
Da Cruz S, Bui A, Saberi S, Lee SK, Stauffer J, McAlonis-Downes M, Schulte D, Pizzo DP, Parone PA, Cleveland DW et al (2017) Misfolded SOD1 is not a primary component of sporadic ALS. Acta Neuropathologica. doi:10.1007/s00401-017-1688-8
David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C (2010) Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol 8:e1000450. doi:10.1371/journal.pbio.1000450
Davies KM, Bohic S, Carmona A, Ortega R, Cottam V, Hare DJ, Finberg JPM, Reyes S, Halliday GM, Mercer JFB et al (2014) Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol Aging 35:858–866. doi:10.1016/j.neurobiolaging.2013.09.034
Dehay B, Bourdenx M, Gorry P, Przedborski S, Vila M, Hunot S, Singleton A, Olanow CW, Merchant KM, Bezard E et al (2015) Targeting alpha-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol 14:855–866. doi:10.1016/S1474-4422(15)00006-X
DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ et al (2008) Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol 65:1074–1080. doi:10.1001/archneur.65.8.1074
Deng HX, Shi Y, Furukawa Y, Zhai H, Fu RG, Liu ED, Gorrie GH, Khan MS, Hung WY, Bigio EH et al (2006) Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci USA 103:7142–7147. doi:10.1073/pnas.0602046103
Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK et al (2009) Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol 8:1150–1157
Domenico FD, Head E, Butterfield A, Perluigi M (2014) Oxidative Stress and proteostasis network: culprit and casualty of Alzheimer’s-like neurodegeneration. Adv Geriatr 2014:14
Double KL, Reyes S, Werry EL, Halliday GM (2010) Selective cell death in neurodegeneration: why are some neurons spared in vulnerable regions? Prog Neurobiol 92:316–329. doi:10.1016/j.pneurobio.2010.06.001
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247. doi:10.1038/35041687
Furukawa Y, Kaneko K, Yamanaka K, O’Halloran TV, Nukina N (2008) Complete loss of post-translational modifications triggers fibrillar aggregation of SOD1 in the familial form of amyotrophic lateral sclerosis. J Biol Chem 283:24167–24176. doi:10.1074/jbc.M802083200
Guareschi S, Cova E, Cereda C, Ceroni M, Donetti E, Bosco DA, Trotti D, Pasinelli P (2012) An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proc Natl Acad Sci USA 109:5074–5079. doi:10.1073/pnas.1115402109
Hare DJ, Double KL (2016) Iron and dopamine: a toxic couple. Brain J Neurol 139:1026–1035. doi:10.1093/brain/aww022
Helferich AM, McLean PJ, Weishaupt JH, Danzer KM (2016) Commentary: alpha-synuclein interacts with SOD1 and promotes its oligomerization. J Neurol Neuromed 1:28–30
Helferich AM, Ruf WP, Grozdanov V, Freischmidt A, Feiler MS, Zondler L, Ludolph AC, McLean PJ, Weishaupt JH, Danzer KM (2015) alpha-synuclein interacts with SOD1 and promotes its oligomerization. Mol Neurodegener 10:66. doi:10.1186/s13024-015-0062-3
Hilton JB, White AR, Crouch PJ (2015) Metal-deficient SOD1 in amyotrophic lateral sclerosis. J Mol Med (Berl) 93:481–487. doi:10.1007/s00109-015-1273-3
Hung LW, Villemagne VL, Cheng L, Sherratt NA, Ayton S, White AR, Crouch PJ, Lim S, Leong SL, Wilkins S et al (2012) The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson’s disease. J Exp Med 209:837–854. doi:10.1084/jem.20112285
Ip P, Mulligan VK, Chakrabartty A (2011) ALS-causing SOD1 mutations promote production of copper-deficient misfolded species. J Mol Biol 409:839–852. doi:10.1016/j.jmb.2011.04.027
Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW (2003) Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 62:389–397
Kacem I, Funalot B, Torny F, Lautrette G, Andersen PM, Couratier P (2012) Early onset Parkinsonism associated with an intronic SOD1 mutation. Amyotroph Lateral Scler 13:315–317. doi:10.3109/17482968.2011.623301
Kato S, Oda M, Tanabe H (1993) Diminution of dopaminergic neurons in the substantia nigra of sporadic amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol 19:300–304
Kerman A, Liu H-N, Croul S, Bilbao J, Rogaeva E, Zinman L, Robertson J, Chakrabartty A (2010) Amyotrophic lateral sclerosis is a non-amyloid disease in which extensive misfolding of SOD1 is unique to the familial form. Acta Neuropathol 119:335–344. doi:10.1007/s00401-010-0646-5
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011) Amyotrophic lateral sclerosis. Lancet 377:942–955. doi:10.1016/S0140-6736(10)61156-7
Koch Y, Helferich AM, Steinacker P, Oeckl P, Walther P, Weishaupt JH, Danzer KM, Otto M (2016) Aggregated alpha-synuclein increases SOD1 oligomerization in a mouse model of amyotrophic lateral sclerosis. Am J Pathol 186:2152–2161. doi:10.1016/j.ajpath.2016.04.008
Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF (2004) A syntaxin 1, Galpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci 24:4070–4081. doi:10.1523/JNEUROSCI.0346-04.2004
Li W, Ye Y (2008) Polyubiquitin chains: functions, structures, and mechanisms. Cell Mol Life Sci 65:2397–2406. doi:10.1007/s00018-008-8090-6
Manno C, Lipari A, Bono V, Taiello AC, La Bella V (2013) Sporadic Parkinson disease and amyotrophic lateral sclerosis complex (Brait–Fahn–Schwartz disease). J Neurol Sci 326:104–106. doi:10.1016/j.jns.2013.01.009
Martinez-Lazcano JC, Montes S, Sanchez-Mendoza MA, Rodriguez-Paez L, Perez-Neri I, Boll MC, Campos-Arroyo HD, Rios C, Perez-Severiano F (2014) Sub-chronic copper pretreatment reduces oxidative damage in an experimental Huntington’s disease model. Biol Trace Elem Res 162:211–218. doi:10.1007/s12011-014-0127-0
Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L (2001) beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci USA 98:12245–12250. doi:10.1073/pnas.211412398
Mather K, Watts FZ, Carroll M, Whitehead P, Swash M, Cairn N, Burke J (1993) Antibody to an abnormal protein in amyotrophic lateral sclerosis identifies Lewy body-like inclusions in ALS and Lewy bodies in Parkinson’s disease. Neurosci Lett 160:13–16
Miller TM, Pestronk A, David W, Rothstein J, Simpson E, Appel SH, Andres PL, Mahoney K, Allred P, Alexander K et al (2013) An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol 12:435–442. doi:10.1016/S1474-4422(13)70061-9
Nilsson MR (2004) Techniques to study amyloid fibril formation in vitro. Methods 34:151–160. doi:10.1016/j.ymeth.2004.03.012
Nishiyama K, Murayama S, Shimizu J, Ohya Y, Kwak S, Asayama K, Kanazawa I (1995) Cu/Zn superoxide dismutase-like immunoreactivity is present in Lewy bodies from Parkinson’s disease—a light and electron-microscopic immunocytochemical study. Acta Neuropathol 89:471–474
Opazo CM, Greenough MA, Bush AI (2014) Copper: from neurotransmission to neuroproteostasis. Front Aging Neurosci 6:143. doi:10.3389/fnagi.2014.00143
Orrell RW, King AW, Hilton DA, Campbell MJ, Lane RJ, de Belleroche JS (1995) Familial amyotrophic lateral sclerosis with a point mutation of SOD-1: intrafamilial heterogeneity of disease duration associated with neurofibrillary tangles. J Neurol Neurosurg Psychiatry 59:266–270
Petrovic N, Comi A, Ettinger MJ (1996) Identification of an apo-superoxide dismutase (Cu, Zn) pool in human lymphoblasts. J Biol Chem 271:28331–28334
Pickles S, Semmler S, Broom HR, Destroismaisons L, Legroux L, Arbour N, Meiering E, Cashman NR, Vande Velde C (2016) ALS-linked misfolded SOD1 species have divergent impacts on mitochondria. Acta Neuropathol Commun 4:43. doi:10.1186/s40478-016-0313-8
Pratt AJ, Shin DS, Merz GE, Rambo RP, Lancaster WA, Dyer KN, Borbat PP, Poole FL 2nd, Adams MW, Freed JH et al (2014) Aggregation propensities of superoxide dismutase G93 hotspot mutants mirror ALS clinical phenotypes. Proc Natl Acad Sci USA 111:E4568–E4576. doi:10.1073/pnas.1308531111
Roberts BR, Lim NKH, McAllum EJ, Donnelly PS, Hare DJ, Doble PA, Turner BJ, Price KA, Lim SC, Paterson BM et al (2014) Oral treatment with Cu-II(atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci 34:8021–8031. doi:10.1523/jneurosci.4196-13.2014
Rotunno MS, Bosco DA (2013) An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. Front Cell Neurosci 7:253. doi:10.3389/fncel.2013.00253
Roudeau S, Chevreux S, Carmona A, Ortega R (2015) Reduced net charge and heterogeneity of pI isoforms in familial amyotrophic lateral sclerosis mutants of copper/zinc superoxide dismutase. Electrophoresis 36:2482–2488. doi:10.1002/elps.201500187
Saccon RA, Bunton-Stasyshyn RKA, Fisher EMC, Fratta P (2013) Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain J Neurol 136:2342–2358. doi:10.1093/brain/awt097
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B et al (2012) Fiji: an open source platform for biological-image analysis. Nat Meth 9:676–682. doi:10.1038/nmeth.2019
Schneeberger A, Tierney L, Mandler M (2015) Active immunization therapies for Parkinson’s disease and multiple system atrophy. Mov Disord. doi:10.1002/mds.26377
Siebert M, Sidransky E, Westbroek W (2014) Glucocerebrosidase is shaking up the synucleinopathies. Brain J Neurol 137:1304–1322. doi:10.1093/brain/awu002
Soon CP, Donnelly PS, Turner BJ, Hung LW, Crouch PJ, Sherratt NA, Tan JL, Lim NK, Lam L, Bica L et al (2011) Diacetylbis(N(4)-methylthiosemicarbazonato) copper(II) (CuII(atsm)) protects against peroxynitrite-induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J Biol Chem 286:44035–44044. doi:10.1074/jbc.M111.274407
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA 95:6469–6473. doi:10.1073/pnas.95.11.6469
Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840. doi:10.1038/42166
Takahashi H, Snow BJ, Bhatt MH, Peppard R, Eisen A, Calne DB (1993) Evidence for a dopaminergic deficit in sporadic amyotrophic lateral sclerosis on positron emission scanning. Lancet 342:1016–1018
Toichi K, Yamanaka K, Furukawa Y (2013) Disulfide scrambling describes the oligomer formation of superoxide dismutase (SOD1) proteins in the familial form of amyotrophic lateral sclerosis. J Biol Chem 288:4970–4980. doi:10.1074/jbc.M112.414235
Valdmanis PN, Belzil VV, Lee J, Dion PA, St-Onge J, Hince P, Funalot B, Couratier P, Clavelou P, Camu W et al (2009) A mutation that creates a pseudoexon in SOD1 causes familial ALS. Ann Hum Genet 73:652–657
Volpicelli-Daley LA, Gamble KL, Schultheiss CE, Riddle DM, West AB, Lee VM (2014) Formation of alpha-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol Biol Cell 25:4010–4023. doi:10.1091/mbc.E14-02-0741
Walker DG, Lue LF, Adler CH, Shill HA, Caviness JN, Sabbagh MN, Akiyama H, Serrano GE, Sue LI, Beach TG et al (2013) Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol 240:190–204. doi:10.1016/j.expneurol.2012.11.020
Wilcox RR (2012) Introduction to robust estimation and hypothesis testing. Elsevier, Oxford
Williams JR, Trias E, Beilby PR, Lopez NI, Labut EM, Bradford CS, Roberts BR, McAllum EJ, Crouch PJ, Rhoads TW et al (2016) Copper delivery to the CNS by CuATSM effectively treats motor neuron disease in SOD(G93A) mice co-expressing the copper-chaperone-for-SOD. Neurobiol Dis 89:1–9. doi:10.1016/j.nbd.2016.01.020
Acknowledgements
Tissues were received from the New South Wales Tissue Resource Centre at the University of Sydney, supported by the Schizophrenia Research Institute and the National Institute of Alcohol Abuse and Alcoholism [NIH (NIAAA) R24AA012725], from the Sydney Brain Bank, which is supported by Neuroscience Research Australia and the University of New South Wales, and from The London Neurodegenerative Diseases Brain Bank, which receives funding from the MRC and as part of the Brains for Dementia Research programme, jointly funded by Alzheimer’s Research UK and Alzheimer’s Society. The authors acknowledge the facilities as well as the scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility (http://ammrf.org.au) node at the University of Sydney. The authors thank Michael Kuligowski (University of Sydney) for excellent technical assistance, Danielle Davies (Brain and Mind Centre) and Sydney Brain Bank for the antibody provision, and Laura Perrin-Verdugier (University of Bordeaux) for assistance with isoelectric focusing experiments.
Author information
Authors and Affiliations
Contributions
BGT, KMD, DJH, and KLD designed the study. BGT, KMD, and KLD applied for all human tissues. KLD raised funds for the study. BGT and KLD gained human research ethics approval. SJGL, PS, BS, CT, CV, CS, SAS, and GMH provided clinical information for all human tissue cases obtained. BGT, KMD, VC, RO, SR, AC, VW, DJH, and KLD designed and performed the experiments. SG and KDS assisted with the experiments. BGT, KMD, VC, RO, SR, AC, VW, HJB, DJH, and KLD analyzed the data. BGT, DJH, and KLD wrote drafts of the manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Funding
This work was supported by funds from the Australian Research Council, the National Health and Medical Research Council of Australia (NHMRC), Parkinson’s NSW (2015 and 2016 seed grants), and the University of Sydney (Biomedical Science, BRIG).
Conflict of interest
The authors declare no competing financial interests or conflicts of interest.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trist, B.G., Davies, K.M., Cottam, V. et al. Amyotrophic lateral sclerosis-like superoxide dismutase 1 proteinopathy is associated with neuronal loss in Parkinson’s disease brain. Acta Neuropathol 134, 113–127 (2017). https://doi.org/10.1007/s00401-017-1726-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-017-1726-6