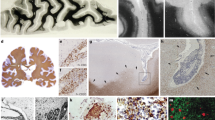
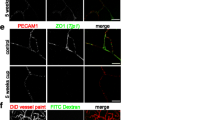
Abstract
Multiple sclerosis (MS) is a chronic neuro-inflammatory disorder, which is marked by the invasion of the central nervous system by monocyte-derived macrophages and autoreactive T cells across the brain vasculature. Data from experimental animal models recently implied that the passage of leukocytes across the brain vasculature is preceded by their traversal across the blood–cerebrospinal fluid barrier (BCSFB) of the choroid plexus. The correlation between the presence of leukocytes in the CSF of patients suffering from MS and the number of inflammatory lesions as detected by magnetic resonance imaging suggests that inflammation at the choroid plexus contributes to the disease, although in a yet unknown fashion. We here provide first insights into the involvement of the choroid plexus in the onset and severity of the disease and in particular address the role of the tight junction protein claudin-3 (CLDN3) in this process. Detailed analysis of human post-mortem brain tissue revealed a selective loss of CLDN3 at the choroid plexus in MS patients compared to control tissues. Importantly, mice that lack CLDN3 have an impaired BCSFB and experience a more rapid onset and exacerbated clinical signs of experimental autoimmune encephalomyelitis, which coincides with enhanced levels of infiltrated leukocytes in their CSF. Together, this study highlights a profound role for the choroid plexus in the pathogenesis of multiple sclerosis, and implies that CLDN3 may be regarded as a crucial and novel determinant of BCSFB integrity.




Similar content being viewed by others
References
Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A (2011) The hedgehog pathway promotes blood–brain barrier integrity and CNS immune quiescence. Science 334(6063):1727–1731
Breij EC, Brink BP, Veerhuis R, van den Berg C, Vloet R, Yan R, Dijkstra CD, van der Valk P, Bo L (2008) Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol 63(1):16–25
Cepok S, Jacobsen M, Schock S, Omer B, Jaekel S, Boddeker I, Oertel WH, Sommer N, Hemmer B (2001) Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain 124(Pt 11):2169–2176
Coisne C, Engelhardt B (2011) Tight junctions in brain barriers during central nervous system inflammation. Antioxid Redox Signal 15(5):1285–1303
Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA (2010) The mouse blood–brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS ONE 5(10):e13741
Frohman EM, Racke MK, Raine CS (2006) Multiple sclerosis–the plaque and its pathogenesis. N Engl J Med 354(9):942–955
Garcia-Vallejo JJ, Van Het HB, Robben J, Van Wijk JA, Van Die I, Joziasse DH, Van Dijk W (2004) Approach for defining endogenous reference genes in gene expression experiments. Anal Biochem 329(2):293–299
Kirk J, Plumb J, Mirakhur M, McQuaid S (2003) Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood–brain barrier leakage and active demyelination. J Pathol 201(2):319–327
Kooi EJ, Prins M, Bajic N, Belien JA, Gerritsen WH, van Horssen J, Aronica E, van Dam AM, Hoozemans JJ, Francis PT, van der Valk P, Geurts JJ (2011) Cholinergic imbalance in the multiple sclerosis hippocampus. Acta Neuropathol 122(3):313–322
Kooij G, Backer R, Koning JJ, Reijerkerk A, van Horssen J, van der Pol SM, Drexhage J, Schinkel A, Dijkstra CD, den Haan JM, Geijtenbeek TB, de Vries HE (2009) P-glycoprotein acts as an immunomodulator during neuroinflammation. PLoS ONE 4(12):e8212
Kooij G, Mizee MR, van Horssen J, Reijerkerk A, Witte ME, Drexhage JA, van der Pol SM, Van Het HB, Scheffer G, Scheper R, Dijkstra CD, Van der Valk P, de Vries HE (2011) Adenosine triphosphate-binding cassette transporters mediate chemokine (C–C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis. Brain 134(Pt 2):555–570
Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE (2008) Structure and function of claudins. Biochim Biophys Acta 1778(3):631–645
Kuijk LM, Klaver EJ, Kooij G, van der Pol SM, Heijnen P, Bruijns SC, Kringel H, Pinelli E, Kraal G, de Vries HE, Dijkstra CD, Bouma G, van Die I (2012) Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol 51(2):210–218
Li Q, Zhang Q, Wang C, Liu X, Qu L, Gu L, Li N, Li J (2009) Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats. J Cell Mol Med 13(9B):4061–4076
Liu P, Jenkins NA, Copeland NG (2003) A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res 13(3):476–484
Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Bruck W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM (2011) Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365(23):2188–2197
Marques F, Sousa JC, Coppola G, Falcao AM, Rodrigues AJ, Geschwind DH, Sousa N, Correia-Neves M, Palha JA (2009) Kinetic profile of the transcriptome changes induced in the choroid plexus by peripheral inflammation. J Cereb Blood Flow Metab 29(5):921–932
Marques F, Sousa JC, Coppola G, Gao F, Puga R, Brentani H, Geschwind DH, Sousa N, Correia-Neves M, Palha JA (2011) Transcriptome signature of the adult mouse choroid plexus. Fluids Barriers CNS 8(1):10
Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE (2012) Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb Perspect Med 2(1):a009381
Murugesan N, Paul D, Lemire Y, Shrestha B, Ge S, Pachter JS (2012) Active induction of experimental autoimmune encephalomyelitis by MOG35-55 peptide immunization is associated with differential responses in separate compartments of the choroid plexus. Fluids Barriers CNS 9(1):15
Ransohoff RM (2009) Immunology: in the beginning. Nature 462(7269):41–42
Ransohoff RM, Engelhardt B (2012) The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 12(9):623–635
Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F (2009) C–C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10(5):514–523
Reijerkerk A, Kooij G, van der Pol SM, Khazen S, Dijkstra CD, de Vries HE (2006) Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. FASEB J 20(14):2550–2552
Reijerkerk A, Lopez-Ramirez MA, Van Het HB, Drexhage JA, Kamphuis WW, Kooij G, Vos JB, van der Pouw Kraan TC, van Zonneveld AJ, Horrevoets AJ, Prat A, Romero IA, de Vries HE (2013) MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: implications for multiple sclerosis. J Neurosci 33(16):6857–6863
Robinson AM, Dorta-Contreras AJ, Lorigados PL (2001) Immune events in central nervous system of early and late onset Alzheimer’s disease patients. Rev Neurol 32(10):901–904
Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE (2007) Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J 21:3666–3676
Song F, Wardrop RM, Gienapp IE, Stuckman SS, Meyer AL, Shawler T, Whitacre CC (2008) The Peyer’s patch is a critical immunoregulatory site for mucosal tolerance in experimental autoimmune encephalomyelitis (EAE). J Autoimmun 30(4):230–237
Steffen BJ, Breier G, Butcher EC, Schulz M, Engelhardt B (1996) ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol 148(6):1819–1838
Vercellino M, Votta B, Condello C, Piacentino C, Romagnolo A, Merola A, Capello E, Mancardi GL, Mutani R, Giordana MT, Cavalla P (2008) Involvement of the choroid plexus in multiple sclerosis autoimmune inflammation: a neuropathological study. J Neuroimmunol 199(1–2):133–141
Willnow TE, Herz J (1994) Homologous recombination for gene replacement in mouse cell lines. Methods Cell Biol 43(Pt A):305–334
Wilson K (2001) Preparation of genomic DNA from bacteria. Current Protocols in Molecular Biology, Chapter 2, Unit 2.4. Wiley
Wolburg H, Paulus W (2010) Choroid plexus: biology and pathology. Acta Neuropathol 119(1):75–88
Wolburg H, Wolburg-Buchholz K, Liebner S, Engelhardt B (2001) Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci Lett 307(2):77–80
Acknowledgments
This work was supported by grants from the Netherlands Organization of Scientific Research (854.10.005 to G. Goverse), the Dutch foundation of Multiple Sclerosis Research (grant MS 12-791, G. Kooij; grant MS 05-567, J. Drexhage; grant 08-642, A. Reijkerkerk), and the Deutsche Forschungsgemeinschaft BL 308/7-4 as well as BL 308/9-1. M. Stuiver, I. C. Meij and D. Müller were supported through a grant by the European Community, FP7 (EUNEFRON 201590). We thank the Netherlands Brain Bank (www.brainbank.nl) for providing the human tissues and Kerstin Sommer for her excellent technical support.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
G. Kooij, K. Kopplin and R. Blasig contributed equally.
D. Müller and I. E. Blasig contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kooij, G., Kopplin, K., Blasig, R. et al. Disturbed function of the blood–cerebrospinal fluid barrier aggravates neuro-inflammation. Acta Neuropathol 128, 267–277 (2014). https://doi.org/10.1007/s00401-013-1227-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1227-1