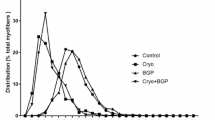
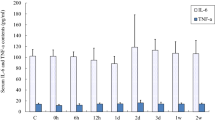
Abstract
The molecular signaling pathway linked to hypertrophy of the anti-gravity/postural soleus muscle after mechanical overloading has not been identified. Using reverse transcription-polymerase chain reaction (RT-PCR), Western blot, and immunohistochemical analyses, we investigated whether the amounts of myocyte enhancer factor (MEF)2C, MEF2D, and myogenin change in the mechanically overloaded soleus muscle after treatment with the calcineurin inhibitor cyclosporine A (CsA). Adult male ICR mice were subjected to a surgical ablation of the gastrocnemius muscle and treated with either CsA (25 mg/kg) or vehicle, once daily. They were killed at 2, 4, 7, 10, and 14 days post-injury. Mechanical overloading resulted in a significant increase in the wet weight and the cross-sectional area of slow and fast fibers of the soleus muscle in placebo-treated mice but not CsA-treated mice. RT-PCR analysis did not show a marked difference in MEF2C and MEF2D mRNA levels in the overloaded soleus muscle in placebo- or CsA-administered mice. After 2 days of mechanical overloading, we observed co-localization of MEF2C and myogenin in several mononuclear cells under both conditions. These MEF2C-positive mononuclear cells also possessed immunoreactivity for c-Met, a satellite cell marker. At 4 days, mechanical overloading induced marked expression of MEF2C but not MEF2D in the subsarcolemmal region in a group of myotubes and/or myofibers. Such a MEF2C-positive region emerged less often in the hypertrophied soleus muscle subjected to the treatment with CsA. At 7 days, we observed many mononuclear cells possessing both MEF2C and myogenin protein in mice treated with CsA, but not the placebo. Our results demonstrated that CsA treatment modulates the amount and cellular localization of MEF2C protein. The modulation of MEF2C by CsA treatment may inhibit the hypertrophic process in the soleus muscle after mechanical overloading.








Similar content being viewed by others
References
Abbott KL, Friday BB, Thaloor D, Murphy TJ, Pavlath GK (1998) Activation and cellular localization of the cyclosporine A-sensitive transcription factor NF-AT in skeletal muscle cells. Mol Biol Cell 9:2905–2916
Black BL, Olson EN (1998) Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol 14:167–196
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS (1998) A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev 12:2499–2509
Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ (1995) Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270:12109–12116
Datta SR, Brunet A, Greenberg ME (1999) Cellular survival: a play in three Akts. Genes Dev 13:2905–2927
Delling U, Tureckova J, Lim HW, De Windt LJ, Rotwein P, Molkentin JD (2000) A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol 20:6600–6611
DeVol DL, Rotwein P, Sadow JL, Novakofski J, Bechtel PJ (1990) Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol 259:E89–E95
Dunn SE, Burns JL, Michel RN (1999) Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem 274:21908–21912
Dunn SE, Chin ER, Michel RN (2000) Matching of calcineurin activity to upstream effectors is critical for skeletal muscle fiber growth. J Cell Biol 151:663–672
Dunn SE, Simard AR, Bassel-Duby R, Williams RS, Michel RN (2001) Nerve activity-dependent modulation of calcineurin signaling in adult fast and slow skeletal muscle fibers. J Biol Chem 276:45243–45254
Florini JR, Ewton DZ, Coolican SA (1996) Growth hormone and the insulin-like growth factor system in myogenesis. Endocrinol Rev 17:481–517
Friday BB, Horsley V, Pavlath GK (2000) Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol 149:657–665
Glass DJ (2003) Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5:87–90
Hinits Y, Hughes SM (2007) Mef2s are required for thick filament formation in nascent muscle fibres. Development 134:2511–2519
Kegley KM, Gephart J, Warren GL, Pavlath GK (2001) Altered primary myogenesis in NFATc3−/− mice leads to decreased muscle size in the adult. Dev Biol 232:115–126
Lai K-MV, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ (2004) Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24:9295–9304
Lazaro JB, Bailey PJ, Lassar AB (2002) Cyclin D-cdk4 activity modulates the subnuclear localization and interaction of MEF2 with SRC-family coactivators during skeletal muscle differentiation. Genes Dev 16:1792–1805
Michel RN, Dunn SE, Chin ER (2004) Calcineurin and skeletal muscle growth. Proc Nutr Soc 63:341–349
Molkentin JD, Firulli AB, Black BL, Martin JF, Hustad CM, Copeland N, Jenkins N, Lyons G, Olson EN (1996) MEF2B is a potent transactivator expressed in early myogenic lineages. Mol Cell Biol 16:3814–3824
Mora S, Pessin JE (2000) The MEF2A isoform is required for striated muscle-specific expression of the insulin-responsive GLUT4 glucose transporter. J Biol Chem 275:16323–16328
Musaró A, McCullagh KJA, Naya FJ, Olson EN, Rosenthal N (1999) IGF-I induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 400:581–585
Musaró A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27:195–200
Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN (2000) Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem 275:4545–4548
Oh M, Rybkin II, Copeland V, Czubryt MP, Shelton JM, Rooij EV, Richardson JA, Hill JA, De Windt LJ, Bassel-Duby R, Olson EN, Rothermel BA (2005) Calcineurin is necessary for the maintenance but not embryonic development of slow muscle fibers. Mol Cell Biol 25:6629–6638
Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S (2002) A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci USA 99:9213–9218
Parsons SA, Wilkins BJ, Bueno OF, Molkentin JD (2003) Altered skeletal muscle phenotypes in calcineurin Aalpha and Abeta gene-targeted mice. Mol Cell Biol 23:4331–4343
Potthoff MJ, Arnold MA, McAnally J, Richardson JA, Bassel-Duby R, Olson EN (2007) Regulation of skeletal muscle sarcomere integrity and postnatal muscle function by Mef2c. Mol Cell Biol 27:8143–8151
Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, Richardson JA, Bassel-Duby R, Olson EN (2007) Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest 117:2459–2467
Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3:1009–1013
Sakuma K, Watanabe K, Totsuka T, Uramoto I, Sakamoto K Sano M (1998) Differential adaptations of insulin-like growth factor, basic fibroblast growth factor and leukemia inhibitory factor in the plantaris muscle of rats by mechanical overloading: an immunohistochemical study. Acta Neuropath (Berl) 95:123–130
Sakuma K, Nishikawa J, Nakao R, Watanabe K, Totsuka T, Nakano H, Sano M, Yasuhara M (2003) Calcineurin is a potent regulator for skeletal muscle regeneration by association with NFATc1 and GATA-2. Acta Neuropath (Berl) 105:271–280
Sakuma K, Nakao R, Aoi W, Inashima S, Fujikawa T, Hirata M, Sano M, Yasuhara M (2005) Cyclosporin A treatment upregulates Id1 and Smad3 expression and delays skeletal muscle regeneration. Acta Neuropath (Berl) 110:269–280
Sakuma K, Nakao R, Yamasa Y, Yasuhara M (2006) Normal distribution of presenilin-1 and nicastrin in skeletal muscle and the differential responses of these proteins after denervation. Biochim Biophys Acta Gen Subj 1760:980–987
Stupka N, Plant DR, Schertzer JD, Emerson TM, Bassel-Duby R, Olson EN, Lynch GS (2006) Activated calcineurin ameliorates contraction-induced injury to skeletal muscles of mdx dystrophic mice. J Physiol 575:645–656
Talmadge RJ, Otis JS, Rittler MR, Garcia ND, Spencer SR, Lees SJ, Naya FJ (2004) Calcineurin activation influences muscle phenotype in a muscle-specific fashion. BMC Cell Biol 5:28
Acknowledgments
This work was supported by a research Grant-in-Aid for Young Scientists B (No. 17700500) from the Ministry of Education, Science, Sports and Culture of Japan, and by the grant for young researcher’s project of Research Center for Future Technology, Toyohashi University of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakuma, K., Akiho, M., Nakashima, H. et al. Cyclosporin A modulates cellular localization of MEF2C protein and blocks fiber hypertrophy in the overloaded soleus muscle of mice. Acta Neuropathol 115, 663–674 (2008). https://doi.org/10.1007/s00401-008-0371-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0371-5