Abstract
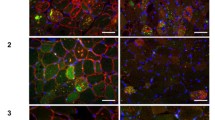
In myotonic dystrophy type 1 (DM1), alternative splicing of ryanodine receptor 1 (RyR1) and sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) genes has been reported. These proteins are essential for maintaining intracellular Ca2+ in skeletal muscle. To clarify involvement of endoplasmic reticulum (ER) stress in DM1 muscles, we examined the activation of ER stress-related proteins by immunohistochemistry, western blot analysis and RT-PCR. In four of five DM1 muscle biopsies, except for a muscle biopsy from a patient with the shortest CTG expansion and no myotonia, increased expression of GRP78 and calnexin, and phosphorylation of PERK and eIF-2α were revealed in fibers with sarcoplasmic masses and in highly atrophic fibers with pyknotic nuclear clumps. Caspase-3 and -7 were also expressed in these fibers. Increased expression of GRP78 in these DM1 muscles was confirmed by western blot analysis. GRP78 mRNA and spliced isoform of XBP1 mRNA were also increased in DM1 muscle biopsies. Furthermore, we demonstrated increased expression of GRP78 in highly atrophic fibers with pyknotic nuclear clumps in all three muscle biopsies from neurogenic muscular atrophies. However, five muscle biopsies from central core disease presumably with disturbed intracellular Ca2+ homeostasis and a muscle biopsy from paramyotonia congenita with myotonia showed no activation of these proteins. Taken together, ER stress is involved in muscle wasting in DM1. However, it seems to be evoked not only by disrupted intracellular Ca2+ homeostasis.



Similar content being viewed by others
References
Benders AAGM, Groenen PJTA, Oerlemens FTJJ, Veerkamp JH, Wieringa B (1997) Myotonic dystrophy protein kinase is involved in the modulation of the Ca2+ homeostasis in skeletal muscle cells. J Clin Invest 100:1440–1447
Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, Hunter K, Stanton VP, Thirion JP, Hudson T, Sohn R, Zemelman B, Snell RG, Rundle SA, Crow S, Davies J, Shelbourne P, Buxton J, Jones C, Juvonen V, Johnson K, Harper PS, Shaw DJ, Housman DE (1992) Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68:799–808
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by precessing the XBP-1 mRNA. Nature 415:92–96
Carpenter S, Karpati G (2001) Pathology of skeletal muscle, 2nd edn. Oxford University Press, New York
Charlet-B N, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA (2002) Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell 10:45–53
Cros D, Harnden P, Pouget J, Pellissier JF, Gastaut JL, Serratrice G (1988) Peripheral neuropathy in myotonic dystrophy: a nerve biopsy study. Ann Neurol 23:470–476
Dubowitz V (1985) Muscle biopsy. A practical approach, 2nd edn. Bailliere Tindall, London
Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4:181–191
Furling D, Marette A, Puymirat J (1999) Insulin-like growth factor I circumvents defective insulin action in human myotonic dystrophy skeletal muscle cells. Endocrinology 140:4244–4250
Furuno K, Goodman MN, Goldberg AL (1990) Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J Biol Chem 265:8550–8557
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic reticulum-resisdent kinase. Nature 397:271–274
Haze K, Yoshida H, Yanagi H, Yura T, Mori K (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell:3787–3799
Ho TH, Charlet-B N, Poulos MG, Singh G, Swanson MS, Cooper TA (2004) Muscleblind proteins regulate alternative splicing. EMBO J 23:3103–3112
Hoffman EP, Lehmann-Horn F, Rüdel R (1995) Overexcited or inactive: ion channels in muscle disease. Cell 80:681–686
Hoozemans JJM, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P, Scheper W (2005) The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol (Berl) 110:165–172
Ikezoe K, Furuya H, Ohyagi Y, Osoegawa M, Nishino I, Nonaka I, Kira J (2003) Dysferlin expression in tubular aggregates: their possible relationship to endoplasmic reticulum stress. Acta Neuropathol (Berl) 105:603–609
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13:1211–1233
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110:1389–1398
Kimura T, Nakamori M, Lueck JD, Pouliquin P, Aoike F, Fujimura H, Dirksen RT, Takahashi MP, Dulhunty AF, Sakoda S (2005) Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in myotonic dystrophy type 1. Hum Mol Genet 14:2189–2200
Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13:385–392
Lyfenko AD, Goonasekera SA, Dirksen RT (2004) Dynamic alterations in myoplasmic Ca2+ in malignant hyperthermia and central core disease. Biochem Biophys Res Commun 322:1256–1266
Maeda M, Taft CS, Bush EW, Holder E, Bailey WM, Neville H, Perryman MB, Bies RD (1995) Identification, tissue-specific expression, and subcellular localization of the 80 and 71 kD forms of myotonic dystrophy kinase protein. J Biol Chem 270:20246–20249
Mankodi A, Takahashi MP, Jiang H, Beck CL, Bowers WJ, Moxley RT, Cannon SC, Thornton CA (2002) Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol Cell 10:35–44
Mori K (2000) Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101:451–454
Nakanishi K, Sudo T, Marishima N (2005) Endoplasmic reticulum stress signaling transmitted by ATF6 mediates apoptosis during muscle development. J Cell Biol 169:555–560
Pall GS, Johnson KJ, Smith GL (2003) Abnormal contractile activity and calcium cycling in cardiac myocytes isolated from dmpk knockout mice. Physiol Genomics 13:139–146
Philips AV, Timchenko LT, Cooper TA (1998) Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 280:737–741
Rao RV, Hermel E, Castro-Obregon S, del Rio G, Ellerby LM, Ellerby HM, Bredesen DE (2001) Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem 276:33869–33874
Ron D (2002) Translational control in the endoplasmic reticulum stress response. J Clin Invest 110:1383–1388
Salviati G, Pierobon-Bormioli S, Betto R, Damiani E, Angelini C, Ringel SP, Salvatori S, Margreth A (1985) Tubular aggregates: sarcoplasmic reticulum origin, calcium storage ability, and functional implications. Muscle Nerve 8:299–306
Sato N, Imaizumi K, Manabe T, Taniguchi M, Hitomi J, Katayama T, Yoneda T, Morihara T, Yasuda Y, Takagi T, Kudo T, Tsuda T, Itoyama Y, Makifuchi T, Fraser PE, George-Hyslop PS, Tohyama M (2001) Increased production of beta-amyloid and vulnerability to endoplasmic reticulum stress by an aberrant spliced form of presenilin 2. J Biol Chem 276:2108–2114
Savkur RS, Philips AV, Cooper TA (2001) Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat Genet 29:40–47
Taneja KL, McCurrach M, Schalling M, Housman D, Singer RH (1995) Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J Cell Biol 128:995–1002
Tews DS, Behrhof W, Schindler S (2005) Expression patterns of initiator and effector caspases in denervated human skeletal muscle. Muscle Nerve 31:175–181
Ueda H, Shimokawa M, Yamamoto M, Kameda N, Mizusawa H, Baba T, Terada N, Fujii Y, Ohno S, Ishiura S, Kobayashi T (1999) Decreased expression of myotonic dystrophy protein kinase and disorganization of sarcoplasmic reticulum in skeletal muscle of myotonic dystrophy. J Neurol Sci 162:38–50
Vattemi G, Engel WK, McFerrin J, Askanas V (2004) Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am J Pathol 164:1–7
Vattemi G, Tomelleri G, Filosto M, Savio C, Rizzuto N, Tonin P (2005) Expression of late myogenic differentiation markers in sarcoplasmic masses of patients with myotonic dystrophy. Neuropathol Appl Neurobiol 31:45–52
Wang JF, Schröder JM (2000) Comparative morphometric evaluation of peripheral nerves and muscle fibers in myotonic dystrophy. Acta Neuropathol (Berl) 99:39–47
Wate R, Ito H, Zhang JH, Ohnishi S, Nakano S, Kusaka H (2005) Expression of an endoplasmic reticulum-resident chaperone, glucose-regulated stress protein 78, in the spinal cord of a mouse model of amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 110:557–562
Wing SS, Haas AL, Goldberg AL (1995) Increase in ubiquitin-protein conjugates concomitant with the increase in proteolysis in rat skeletal muscle during starvation and atrophy denervation. Biochem J 307:639–645
Wu S, Ibarra M CA, Malicdan MCV, Murayama K, Ichihara Y, Kikuchi H, Nonaka I, Noguchi S, Hayashi YK, Nishino I (2006) Central core disease is due to RYR1 mutation in more than 90% of patients. Brain 129:1470–1480
Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL (2000) ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6:1355–1364
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891
Acknowledgments
Parts of this work were supported by a Research Grant (17A-10) for nervous and mental disorders from the ministry of Health, Labor and Welfare, Japan, to J.K. and S.S., and by Grants-in Aid for Scientific Research from the Japan Society for the Promotion of Science to M.P.T. We are grateful to Miss S. Nagae for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikezoe, K., Nakamori, M., Furuya, H. et al. Endoplasmic reticulum stress in myotonic dystrophy type 1 muscle. Acta Neuropathol 114, 527–535 (2007). https://doi.org/10.1007/s00401-007-0267-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-007-0267-9