Abstract.
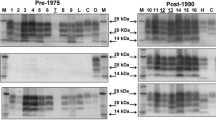
A panel of ruminant brain tissues were subjected to a Western immunoblotting technique using two monoclonal antibodies (mAbs). The resultant prion protein (PrP) glycoforms showed that three distinctions can be made between natural ovine scrapie cases and sheep experimentally inoculated with bovine spongiform encephalopathy (BSE). Differentiation between BSE-infected cattle and natural cases of sheep scrapie was also possible using these two antibodies. There were subtle differences in the molecular weight positions of the di-glycosylated, mono-glycosylated and unglycosylated forms of the abnormal PrP (PrPSc) associated with these ruminant transmissible spongiform encephalopathies. In particular, a distinct difference for the unglycosylated protein band was observed. For ovine scrapie samples, this band was noticeably of a higher molecular weight than that found for brain samples from the Romney and Cheviot breed sheep infected with BSE and, to a lesser degree, higher than that observed for bovine BSE samples. Using the comparison of glycoform ratios, the technique provided a distinction between the sheep experimentally infected with BSE and natural cases of sheep scrapie but did not provide a distinction between natural cases of bovine BSE and ovine scrapie. The sheep-passaged CH1641 scrapie strain gave molecular weights similar to, but not identical to BSE, and a glycoform ratio similar to ovine scrapie cases. The SSBP1 experimental scrapie strain gave molecular weights that were akin to natural scrapie cases but the glycoform ratio was different to that found for all the other samples. When mAb P4 was substituted for mAb 6H4 in the technique, only the natural scrapie samples and SSBP1 gave strong signals. BSE in sheep and the CH1641 strain gave weak reactions and PrPSc from BSE-infected cattle could not be detected at all. The results suggest that this combination of molecular weight and glycoform ratio analyses, and differentiation with two specific antibodies could be used to provide a possible screening test for BSE in the UK sheep flock, if confirmed as accurate by bioassay and lesion profile analysis in mice inoculated with brain tissue from suspect field cases.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Stack, M., Chaplin, M. & Clark, J. Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep-passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol 104, 279–286 (2002). https://doi.org/10.1007/s00401-002-0556-2
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00401-002-0556-2