Abstract
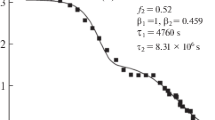
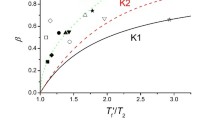
Cumulative density-relaxation data from the literature (following a thermal quench or during a constant rate of cooling) for polystyrene are analyzed. Consideration is given to four well-known models for the relaxation time as a function of temperature and fictive temperature, starting with the seminal modeling proposed by Tool in 1946. Among the numerous experimental results, those presented by Greiner & Schwarzl in 1984 are the most extensive, including their notable result that, under constant-cooling rate, the apparent glass-transition temperature (Tg,apparent) varies linearly with the log of the cooling rate (qc). In the present paper, it is established by detailed numerical analysis that Tool's model is the one which replicates this linear dependence between Tg,apparent and log qc. In turn, this implies that the temperature dependence of the equilibrium relaxation time (or the temperature shift factor) is purely exponential below the glass-transition temperature. This behavior is also shown to describe available experimental data in the literature (all below the nominal glass-transition temperature) for the stress relaxation of polycarbonate and the dielectric compliance of polyvinyl acetate.










Similar content being viewed by others
References
Abramowitz M, Stegun IA (1965) (editors) Handbook of mathematical functions. Dover, New York
Adachi K, Kotaka T (1982) Volume and enthalpy relaxation in polystyrene. Polym J 14(12):959–970
Braun G, Kovacs AJ (1963) Glass transition in powdered polystyrene. Phys Chem Glasses 4(4):152–160
Colby RH (2000) Dynamic scaling approach to glass formation. Phys Rev E 61(2):1783–1792
Dirac PAM (1958) Principles of quantum mechanics, 4th edn. Oxford UP, London
Eighth IDMRCS (2017) (International Discussion Meeting on Relaxations in Complex Systems) in Wista, Poland; July 23-28
Ferry JD (1980) Viscoelastic properties of polymers, 3rd edn. Wiley, New York
Fulcher GS (1925) Analysis of recent measurements of the viscosity of glasses. J Am Ceram Soc 8(6):339–355
Gao S, Simon SL (2015) Measurement of the limiting fictive temperature over five decades of cooling and heating rates. Thermochim Acta 603:123–127
Goldbach G, Rehage G (1967) Die Volumenretardation des Polystyrols nach Druck-und Temperatursprüngen. Rheol Acta 6(1):30–53
Greiner R, Schwarzl FR (1984) Thermal contraction and volume relaxation of amorphous polymers. Rheol Acta 23(4):378–395
Greiner R, Schwarzl FR (1989) Volume relaxation and physical aging of amorphous polymers. I. Theory of volume relaxation after single temperature jumps. Colloid Polym Sci 267(1):39–47
Hieber CA (2022) Correlations for the segmental relaxation time and the second-newtonian viscosity of polystyrene. Rheol Acta (pending)
Hodge IM (1987) Effects of annealing and prior history on enthalpy relaxation in glassy polymers. 6 Adam-Gibbs formulation of nonlinearity. Macromolecules 20(11):2897–2908
Hodge IM (1994) Enthalpy relaxation and recovery in amorphous materials. J Non-Cryst Solids 169(3):211–266
Höhne GWH, Schick C (2015) Chip calorimetry. Thermochim Acta 603:1
Hozumi S, Wakabayashi T, Sugihara K (1970) Volume retardation of polystyrene. Polym J 1(6):632–638
Hutchinson JM (1995) Physical aging of polymers. Prog Polym Sci 20(4):703–760
Koh YP, Simon SL (2018) Enthalpy recovery of ultrathin polystyrene film using Flash DSC. Polymer 143:40–45
Kohlrausch P (1847) Ueber das Dellmann'sche Elektrometer. Ann Phys Chem 72(11):353–405
Kovacs AJ (1958) La contraction isotherme du volume des polymères amorphes. J Polym Sci 30(3):131–147
Kovacs AJ (1963) Transition vitreuse dans les polymères amorphes. Etude phénoménologique. Fortschr Hochpolym-Forsch 3:394–507
Lee HHD, McGarry FJ (1990) Glass and β transitions of atactic polystyrenes monitored by volume dilatometry. J Macromol Sci-Phys B29(2 & 3):185–202
Lighthill MJ (1959) Introduction to Fourier analysis and generalized functions. Cambridge UP, London
McKenna GB (1989) In: Booth C, Price C (eds) Comprehensive polymer sci, vol 2. Pergamon, Oxford, pp 311–362
Moynihan CT, Macedo PB, Montros CJ, Gupta PK, DeBolt MA, Dill JF, Dom BE, Drake PW, Easteal AJ, Elterman PB, Moeller RP, Sasabe H, Wilder JA (1976) Ann N Y Acad Sci 279:15–35
Narayanaswamy OS (1971) A model of structural relaxation in glass. J Am Ceram Soc 54:491–498
Nelder JA, Mead R (1965) A simplex method for function minimization. Comput J 7(4):308–313
Ngai KL (2011) Relaxation and diffusion in complex systems. Springer, New York
O’Connell PA, McKenna GB (1999) Arrhenius-type temperature dependence of the segmental relaxation below Tg. J Chem Phys 110(22):11054–11060
Oels H-J, Rehage G (1977) Pressure-volume-temperature measurements on atactic polystyrene. A thermodynamics view. Macromolecules 10(5):1036–1043
Prest WM Jr, Penwell RC, Luca DJ, Roberts FJ Jr (1980) Volume and enthalpy recovery in the same polystyrene glass. Am Chem Soc Polymer Preprints 21(2):10–11
Richardson MJ, Savill NG (1975) Derivation of accurate glass transition temperatures by differential scanning calorimetry. Polymer 16(10):753–757
Richardson MJ, Savill NG (1977) Volumetric properties of polystyrene: influence of temperature, molecular weight and thermal treatment. Polymer 18(1):3–9
Roland CM (2011) Viscoelastic behavior of rubbery materials. Oxford UP, Oxford
Rusch KC (1968) Time-temperature superposition and relaxation behavior in polymeric glasses. J Macromol Sci A B2(2):179–204
Schawe JEK (2015) Measurement of the thermal glass transition of polystyrene in a cooling rate range of more than six decades. Thermochim Acta 603:128–134
Scherer GW (1984) Use of the Adam-Gibbs equation in the analysis of structural relaxation. J Am Ceram Soc 67(7):504–511
Seventh IDMRCS (2013) (International Discussion Meeting on Relaxations in Complex Systems) in Barcelona, Spain; July 21-26 (2013). Proc J Non-Cryst Solids 407:1–502
Simon SL, Sobieski JW, Plazek DJ (2001) Volume and enthalpy recovery of polystyrene. Polymer 42(6):2555–2567
Struik LCE (1978) Physical aging in amorphous polymers and other materials. Elsevier, Amsterdam
Struik LCE (1990) Internal stresses, dimensional instabilities and molecular orientations in plastics. Wiley, Chichester
Tammann G, Hesse W (1926) Die Abhängegkeit der Viskosität von der Temperatur bei unterkühlten Flüssigkeiten. Z Anorg Allg Chem 156:245–257
Tool AQ (1946) Relaxation between inelastic deformability and thermal expansion of glass in its annealing range. J Am Ceram Soc 29(9):240–253
Vogel H (1921) Das Temperatur-abhängigkeitsgesetz der Viskosität von Flüssigkeiten. Phys Z 22:645–646
Williams G, Watts DC (1970) Non-symmetrical dielectric relaxation behavior arising from a simple empirical decay function. Trans Faraday Soc 66:80–85
Williams ML, Landel RF, Ferry JD (1955) The temperature dependence of relaxation mechanisms in amorphous polymers and other glass-forming liquids. J Am Chem Soc 77(14):3701–3707
Zhao J, McKenna GB (2012) Temperature divergence of the dynamics of a poly(vinyl acetate) glass: Dielectric vs. mechanical behaviors. J Chem Phys 136:154901
Zoller P, Bolli P, Hersche E, Foppa U (1976) Das p-v-T Diagramm von Polystyrol. Kunststoffe 66(6):363–367
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to Professor K.K. Wang, Director of the Cornell Injection Molding Program (1974-2000); also in remembrance of Professor S.F. Shen and Dr. V.W. Wang, and with thanks to Professor W.R. Jong of Chung Yuan Christian University, Taiwan.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Governing equations
Making use of Equations (1) and (7) from the text, we can write the governing equation for ϕ as
where
For the case of a thermal quench (from Ti to Tbath at t = 0), we have that qc is a Dirac delta function (Dirac 1958; Lighthill 1959) of magnitude
such that the solution for t > 0+ is simply
where
Or, if the KWW (Kohlrausch 1847; Williams and Watts 1970) parameter is introduced, (46) becomes
where β typically lies between 0 and 1. In this case, the equivalent differential equation becomes
for t > 0+, subject to the initial condition
For the constant-cooling-rate case, with qc a constant in (43) & (44), the closed-form solution (with KWW parameter included) is simply
where
and Q(t) as defined in (47). Alternatively, the governing differential equation in this case becomes
subject to the initial condition:
In particular, the factor βQβ-1 common to both (49) and (53) reduces to unity when β=1 (i.e., when the KWW approximation becomes an identity).
In relating the computational variable ϕ (≡ Teff - T) to the experimentally determined variable χ (≡ υ - υ∞), we make use of Equation (9) in the text which directly relates the two via the constant Δα* defined in (10). That is,
measures the temperature sensitivity of the specific volume (throughout, it is tacitly assumed that the pressure is atmospheric). In general, α*(T) tends to be a constant, αl* (say), at sufficiently high T and a correspondingly smaller constant, αg* , at sufficiently low T.
An underlying assumption in the simulation is that the specific volume υ(t) responds instantaneously to changes in T(t) via the temperature sensitivity αg*. For example, in the case of a thermal quench, with T(t) undergoing a step change of magnitude ΔT down from Ti to Tbath , it is assumed that the corresponding instantaneous change in υ(t) is
whereas υ∞(t), the equilibrium specific volume, undergoes a change of
Accordingly, since υ = υ∞ initially (when the system is in equilibrium), it follows that, at t = 0+,
that is,
where use has been made of (8), (10) and (50). That is, (58) is the same as (9), which is assumed to apply at all t.
Furthermore, if we now consider the commonly-employed non-dimensional variable δ which, for the thermal-quench case, is typically defined as
it then follows from (8) and (9) that
Typically, the coefficient in (60) is taken to be a constant, denoted as Δα, where
and
is analogous to Δα* in (10).
Of course, if the overall temperature range of Tbath is sufficiently small such that the variation in υ∞(Tbath) is negligible, it is then reasonable to treat the coefficient in 60) as constant. However, in the present study we include data in which υ∞(Tbath) varies by almost 10%. {For example, with dυ/dT ≈ 5.5 × 10−4cm3/gm°C (from third column of Table 10) and Tbath ranging from -52°C to about 100°C, it follows that υ∞ (T) varies by about 0.084 cm3/gm overall.} Accordingly, in fitting the data, it will be more accurate to fit the data in terms of its dimensional form, χ, rather than its non-dimensional form, δ, thus avoiding the inaccuracy of treating the coefficient in (60) as a constant. This is what has been done in the present investigation.
Appendix 2: Value of ∆α* for polystyrene
Listed in Table 10 are relevant data sources from the literature for the case of polystyrene. As indicated, many of the cited results are based upon thermal-quench data which have been replotted in terms of “aging time” (ta) measured from when the specimen was quenched. In particular, such plots are given directly in Lee and McGarry (1990) and Struik (1978), whereas the remaining entries for specified ta have been obtained by cross-plotting the underlying thermal-quench data. Based upon the cumulative results, it seems that a reasonable representative value for ∆α* is 3.3 × 10-4 cm3/(gm°C), which is what has been employed throughout the simulated results in the present investigation.
Numerically, the present calculations for both the thermal-quench and constant-cooling-rate cases have been done mainly in terms of the differential-equation formulation, using a fourth-order Runge Kutta (formula 25.5.10 from Abramowitz and Stegun 1965). The results have been corroborated in many instances by using the integral formulation in terms of the simple trapezoidal rule (formula 25.4.1 from Abramowitz and Stegun 1965).
Rights and permissions
About this article
Cite this article
Hieber, C.A. Modeling the density relaxation of polystyrene. Rheol Acta 61, 523–538 (2022). https://doi.org/10.1007/s00397-022-01344-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00397-022-01344-1