Abstract
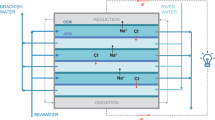
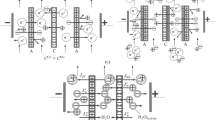
Electrodialysis of mixed salt solutions, sodium chloride and sodium sulfate, and sodium chloride and sodium nitrate, was carried out in the presence of α-cyclodextrin using commercial anion-exchange membranes. It was confirmed by several methods that the compound existed in the membrane matrix when the membrane had been immersed in its aqueous solution, though the molecular weight of α-cyclodextrin is relatively high. In electrodialysis, sulfate ions, large and strongly hydrated anions, easily permeated through the membranes and nitrate ions, less hydrated anions, permeated with difficulty through the membranes in the presence of α-cyclodextrin. Because α-cyclodextrin is a hydrophilic compound, which has many ether and alcoholic groups, the hydrophilicity of the anion-exchange membranes is thought to increase. Thus, sulfate ions easily permeate and nitrate ions permeate with difficulty. This proves that the hydrophilicity of the anion-exchange membranes controls permselectivity between anions through the membranes.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 8 August 2000 Accepted: 24 October 2000
Rights and permissions
About this article
Cite this article
Sata, T., Kawamura, K., Higa, M. et al. Electrodialytic transport properties of anion-exchange membranes in the presence of α-cyclodextrin. Colloid Polym Sci 279, 413–419 (2001). https://doi.org/10.1007/s003960000459
Issue Date:
DOI: https://doi.org/10.1007/s003960000459