Abstract
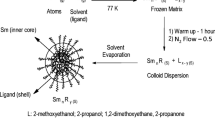
Nickel colloids were prepared by codeposition of the metal with several organic solvents: acetone, ethanol, 2-propanol, 2-methoxyethanol and 1,2-dimethoxyethane at 77 K. The stabilities of the colloids and fine powders were measured. The metallic films and active solids were obtained by evaporation under vacuum at room temperature. The Ni-2-methoxyethanol colloids are stable over three months at room temperature, and the UV-VIS absorption spectra of the most stable colloids were obtained.
A chemical characterization of Ni solids was carried out by several techniques such as elemental analysis, FT-IR spectroscopy, differential scanning calorimetry (DSC), and thermogravimetry (TGA).
The IR studies show the presence of the solvent in the solids, which was confirmed by microanalysis. From TGA, DSC, and TGA-FTIR the metal-solvent was studied. From TGA, the kinetic parameters of decomposition reaction were calculated using the Freeman and Carroll equations.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 6 June 2000 Accepted: 6 September 2000
Rights and permissions
About this article
Cite this article
Cárdenas, G., Acuña, J. Nickel nanoparticles and solids using organic solvents. Colloid Polym Sci 279, 442–448 (2001). https://doi.org/10.1007/s003960000440
Issue Date:
DOI: https://doi.org/10.1007/s003960000440