Abstract
Aqueous liquid suspensions of high aspect ratio 2D clay nanosheets were investigated using small angle X-ray scattering (SAXS). The high aspect ratio of synthetic fluorohectorite clays allows for investigation of liquid crystalline orientational order for relatively large nanosheet spacings, in the range which can produce structural coloration, thus providing two handles for determining the nanosheet spacings: SAXS and visible color. Various clay concentrations were investigated, and good agreement with previous work on structural coloration from such suspensions was obtained. Particular attention is given to the confinement caused by the container geometry, where both cylindrical and flat confinements were investigated. In both cases, the SAXS data suggest coherent regions that have a nematic inter-orientational distribution that surprisingly is linked to the container geometry, which apparently determines the efficiency of packing of the suspension. For both geometries, the analysis suggests that these coherent regions have a 1D lamellar periodic intra-structure with uniform nanosheet spacing determined by the clay concentration and a typical correlation length in the range of 200 to 500 nm.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Clays have always been used as a material by humans [1]. Clays are 2D nano-lamellar materials [2, 3], and as such, they offer an array of possible applications, for instance, as barrier coatings [4,5,6,7,8], hydrogels [9,10,11], capture and storage of gasses [12,13,14], as well as photonic crystals producing structural colors [15].
Almost a century has passed since Langmuir’s experiments discussing clay colloidal suspensions in terms of liquid crystal structures [16]. Such experiments were the basis for Onsager’s seminal paper on the first purely entropy-driven nematic phase transition [17]. Later several experimental studies of colloidal platelet suspensions have been performed, for instance by Lekkerkerker and collaborators [18,19,20]. This has inspired renewed interest in anisometric clay platelets in suspensions and their liquid crystalline phase behavior [20,21,22,23,24]. When the concentration of suspended clay nanosheets is increased, typically the system forms a gel [23, 25,26,27] with a yield stress preventing flow [24]. It has been debated how gelation might interfere with the formation of liquid-crystalline structuring [28,29,30,31,32].
Clay-based platelets can come in two versions [33], either as stacked nano-lamellar (> 20 nm thick) flat particles [34,35,36,37], or as delaminated thin nanosheets (ca. 1 nm for a monolayer) in aqueous suspension [38]. In the former case, gravity in combination with container confinement and particle to wall anchoring [39] are responsible for sorting clay particles, allowing for observation of the nematic-isotropic transition in the same sample tube [34,35,36,37, 40].
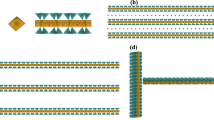
High aspect ratio (lateral size of 20 μm and thickness of 1 nm) [41] synthetic sodium fluorohectorite (NaHec) with a nominal formula unit of \({\left[\text{N}{\text{a}}_{0.5}\right]}^{\text{i}\text{n}\text{t}\text{e}\text{r}}{\left[\text{M}{\text{g}}_{2.5}\text{L}{\text{i}}_{0.5}\right]}^{\text{o}\text{c}\text{t}} {\left[\text{S}{\text{i}}_{4}\right]}^{\text{t}\text{e}\text{t}} {\text{O}}_{10}{\text{F}}_{2}\) [42] can delaminate into a mixture of nematic domains and metastable colloidal accordions where the single layers are separated to uniform distances defined by the amount of water and electrostatic interactions governing the separation [2, 38]. These lamellae stacks bear similarities with a smectic liquid-crystalline phase [43]. By adjusting the ratio of NaHec/water, it is even possible to separate the single layers (SGL) to distances comparable with the wavelengths of visible light allowing for structural colors. Fixation of these tuned layer-separation distances in (polymeric) matrices could open the way to tunable bright coloration for nowadays applications. Recently, it was shown that the brightness is significantly increased for dispersions of double layered clay nanosheets (DBL) [15]. Figure S1 in the supplementary information shows typical reflectance spectra in the visible range for the herein investigated DBL suspensions, in agreement with literature [15]. The DBLs were obtained from NaHec, following a protocol which includes a partial ion exchange of interlayer sodium cations with cesium cations leading to an ordered interstratification of Cs+- and Na+-interlayers. As Cs+ has a substantial lower hydration enthalpy than Na+ only along the later interlayers delamination is observed upon immersion of the ordered interstratification into deionized water (Fig. 1).
Production of SGL and DBL clay suspensions. The swelling behavior of NaHec is modified when Na+ (yellow) is partially exchanged with Cs+ (green). NaHec delaminates into single lamellae (SGL) upon immersion in water. In contrast, ordered interstratification enables delamination of double layered clay nanosheets (DBL) pinned together by cesium ions. Additionally, the general structure of a silicate layer in NaHec is shown (more details in Fig. S2 in the supplementary information)
NaHec changes its swelling behavior in dependence of the volume fraction: The first steps of layer separation occur via discrete hydration steps (crystalline swelling, regime I). With further uptake of water, a gel phase is obtained, in which the layers of a tactoid are still positionally strongly correlated and localized in well-defined distances d (Wigner crystal, regime II). For even lower volume fractions, a transition to a more disordered regime takes place due to the increasing loss of correlation between the adjacent platelets (osmotic swelling regime III, lattice melting) [42]. Because of the huge lateral expansion, in all phases individual clay nanosheets are forced in coplanar arrangement in statistically oriented domains originating from the pristine non-hydrated layered crystals. The individual layers in the coplanar arrangement, however, partially translate, tilt and wrinkle to gain conformational entropy resulting in certain deviations from perfect coplanarity. This leads to a significant reduction of the coherent correlation length in the ordered domains. Nevertheless, the swelling regime is still characterized by a power law scaling with the volume fraction ϕ, e.g., the layer separation distance d scales with ϕ−x. Previous small angle X-ray scattering (SAXS) measurements indicate a crossover from a power law exponent x = −0.66 at low ϕ (in the range 0.2 to 2 vol%) to x = −1 at higher ϕ (in the range 2 to 10 vol%) for SGL suspensions [42], whereas observations of structural coloration from DBL suspensions at low ϕ indicated a power law of x = −0.84 (in the ϕ range 0.67 to 1.34 vol%) [15]. Due to the extreme aspect ratio of the silicate layers, an isotropic suspension with freely rotating individual nanosheets can only be obtained for very low concentrations (ϕ < 0.0001).
We note that in the previous works different confinement geometries were used for the suspensions, cylindrical capillaries [42] or flat cuvettes [15], respectively. However, they were not directly compared and, as demonstrated in this paper, this can impact on the results of the studies. Therefore, in the present work, SAXS is used to investigate highly diluted SGL and DBL suspensions in different sample container geometries in a concentration regime allowing for structural color. We find that the confinement geometries yield power law ϕ behaviors that are consistent with the dependencies reported in previous publications [15, 42].
Samples and experiments
Fluorohectorite
Melt synthesis and long-term annealing of the synthetic sodium fluorohectorite \(({\left[\text{N}{\text{a}}_{0.5} \right]}^{\text{i}\text{n}\text{t}\text{e}\text{r}}{\left[\text{M}{\text{g}}_{2.5}\text{L}{\text{i}}_{0.5}\right]}^{\text{o}\text{c}\text{t}}{\left[\text{S}{\text{i}}_{4}\right]}^{\text{t}\text{e}\text{t}}{\text{O}}_{10}{\text{F}}_{2}\)) (NaHec) was performed according to literature procedures [44]. The material featured a cation exchange capacity of 1.27 mmol/g and a density of 2.73 g/cm3. CsCl (ReagentPlus, ≥ 99.9%), NaCl (EMSURE ACS, ISO, Reag. Ph. Eur, ≥ 99.5%) were used as obtained. The clay suspensions were diluted to the targeted concentrations using stock solutions (ϕ = 1 vol% for SGLs, and ϕ = 2 vol% for DBL), which were prepared by spontaneous delamination of NaHec tactoids in water due to the repulsive interactions between the interlayers. The average platelet dimensions were ⁓ 20 μm in lateral size and 1.0 nm in thickness for the SGL and 2.1 nm for the DBL (see Fig. S3 in supplementary information). The stock solutions appear fluid and transparent to the eye, suggesting that the delamination was homogeneous. Before the transfer to the sample containers, the diluted suspensions were put in an overhead shaker (Trayter D, IKA) at 80 rpm for 16 h. It was confirmed that the diluted suspensions were not gelled, as seen in Fig. S4 in the supplementary information. The clay suspensions were inserted by means of a syringe (0.8 mm ø needle) in the respective sample containers, either a capillary (Hilgenberg, diameter 2 mm, product code: 4,007,620) or a flat cell (Hellma quartz cuvette with 1 mm thickness and 9.5 mm width, product code: Z803111). Afterwards, to check for structural coloration photos were taken using an iPhone 13 Pro camera as shown in Fig. 2.
Structural coloration from DBL and SGL suspensions. The DBL suspensions in (a) cuvettes (1.0 mm × 9.5 mm) (1.15 vol%, 0.92 vol%, and 0.68 vol%, respectively) and (c) capillary (0.48 vol%, 2.0 mm ø). The SGL suspensions in (b) cuvettes (0.48 vol%, 0.40 vol%, and 0.28 vol%, respectively) as they were measured at the ESRF DUBBLE beamline. The DBL suspensions display bright structural colors in contrast to the very weak colors for the SGL samples
Small angle X-ray scattering
The SAXS experiments were performed at the European Synchrotron Radiation Facility (ESRF, BM26 DUBBLE beamline). For the X-ray scattering pattern acquisition, a fixed wavelength λ = 1.03 Å was used, with a beam size of 0.8 mm × 3.2 mm (VxH). The scattering intensities were recorded by a Pilatus 1 M detector (Dectris, pixel size 172 × 172 μm [2], with an active area of 168 mm × 179 mm) placed at a distance from the sample of 9.34 m, resulting on a q-range from 2.2·10−2 to 1.3 nm−1 (where q = 4π/λ sin(θ) and 2θ is the scattering angle). A simple representation of the irradiation geometry and the characteristic 2D SAXS images are shown in Fig. 3. Due to the container orientational confinement, it is important to keep in mind the direction of the beam relative to the clay nanosheet orientations. For the cuvettes, the setup at the beamline blocked the beam at the lowest q values.
Irradiation geometry, sample container, and characteristic 2D SAXS images. A simple representation of the beam direction for the two investigated geometries, cuvettes (a) and capillaries (b). Examples of obtained diffractograms are shown for both geometries. The X-ray path length through the suspension was 9.5 mm for the cuvettes, whereas for the capillaries it was 2.0 mm
The 2D diffractograms were converted by 360° azimuthal integration into their 1D representations (\(I\left(q\right)\;\mathrm{vs}.\;q\)) following the beamline’s standard procedure. 1D data were fit using the Förster model [45], previously used for similar studies on clay systems [42]. In this model, the intensity \(I\left(q\right)\) is given by:
where \(b\) is the scattering contrast between the clay nanosheets and the solvent, \(\rho\) the number density of the layers, \(q\) the scattering vector, and \(P\left(q\right)\) the form factor of disks. The terms inside the square brackets constitute the structure factor, where \(Z\left(q\right)\) is the lattice factor determining the distance \(d\) of adjacent nanosheets in the liquid, \(G\left(q\right)\) is the Debye–Waller factor, and \(\beta \left(q\right)\) is a polydispersity factor [45].
In order to crosscheck the Förster model, we also tested the more commonly used SasView [46]. See supplementary information.
Results and discussion
The experimental results of the SAXS are summarized in Fig. 4, where the plots for DBL and SGL suspensions in cuvettes and capillaries are shown with their respective fits using the Förster model (disks separated by distance d).
1D I(q) vs. q for DBL and SGL suspensions. The colored plots in the top row show the 1D diffractograms for DBL suspensions and the corresponding fits. The agreement between both is reasonable. The bottom black and white plots show the 1D diffractograms for SGL suspensions and the corresponding fits. Curves are shifted vertically with an arbitrary scaling factor for clarity
The results for the distances d are summarized in Fig. 5 and can be found as well in Table S1 in the supplementary information. The estimated correlation lengths are in the range of 200 to 500 nm. Note that as shown in Fig. S5 for the capillary case, the contribution from the scattering volume is related to the packing of the suspension in the central region of the capillary, and the packing itself is a result of the wall anchoring.
The behavior of the d-spacing as a function of sheet type (SGL or DBL), concentration and cell geometry is shown in Fig. 5.
The log-log plots in Fig. 5 suggest power laws for d vs. ϕ for DBL and SGL suspensions. Note that somewhat surprisingly the power law exponent depends on the geometry of the sample container, being significantly higher for cuvettes than for capillaries. These results are, however, in very good agreement with previous results obtained from DBL structural coloration in cuvettes [15] and with SAXS studies of SGL suspensions in capillaries [42]. Modeling using SasView gives approximately the same result (Fig. S6 in supplementary information).
Comparison of the 2D patterns, right column of Fig. 6, shows that the data taken in cuvettes are more anisotropic than in capillaries. Thus, the (local) spatial and orientational distribution of high aspect ratio nanosheets in suspension is largely defined by the border slip conditions induced by the confinement geometry of the sample container (giving the boundary conditions for swelling). Whereas for the cuvette there is only one main orientation direction, for the capillaries, more than one main orientation direction is observed. The texture of the samples is better visible and quantifiable in the azimuthal plots, also given in Fig. 6. The liquid crystalline domains are better oriented in flat cuvettes as can be seen from the significantly smaller FWHM of the Lorentzians. The FWHM are ca. 12° and ca. 50° for blue DBL (1.5 vol%) sample in cuvette and capillary, respectively. See Figs. S7 and S8 in supplementary information for the azimuthal angle plots for all DBL in cuvettes and capillaries, as well as for SGL case.
Normalized intensity vs. azimuthal angle for blue DBL suspensions in different sample containers. On the left column are the azimuthal plot for blue DBL in cuvette (top) and capillary (bottom). As expected, there is a sharp alignment (texture) in the cuvette case. On the right column are enlarged image sections of the respective 2D SAXS patterns
Referring to Table S1, the relative displacement (Δd/d) is noticeably smaller for DBL (in average 12 ± 1.5%) when compared to SGL (in average 18 ± 2.5%), and it is similar between the two geometries. This can possibly be associated with differences in bending rigidity, which is higher for DBL than for SGL [47]. Our observed relative displacement values are higher than 10% which is above the Lindemann criterion [48] for lattice melting as discussed previously by Rosenfeldt et al. [42]. Swelling to structural coloration is in the melted regime, in which the platelets are still correlated in plane, but are not anymore ideally stiff and strictly coplanar. Dislocations by sliding or tilting of planes as well as bending of sheets may become important and can influence the local order of colloidal accordions. These transitions most likely influence the spatial and the orientational distribution of the platelets.
For capillary containers, the behavior of the d-spacing as a function of the volume fraction follows a power law previously shown to be ϕ−1 for concentrated SGL suspensions (Wigner crystals, d < 30 nm, and ϕ−0.66 for more dilute suspensions, d > 30 nm) [42]. In the present work, these power law dependencies are shown to be closer to ϕ−1 for DBL samples compared to SGL samples, which we attribute to the higher stiffness of DBL nanosheets. Also, it seems evident that the power law dependency is closer to ϕ−1 for cuvette than for capillary containers, presumably because nanosheets may “pack” more efficiently in flat cuvettes.
The internal domain nanosheet d-spacing increases with decreasing ϕ, as consistent with previous observations [15, 42]. The measured d-spacings of samples in capillaries are smaller than the ones in cuvettes for all cases. This difference can be up to 15% and is consistent for both, SGL and DBL, due to different domain alignments in the capillaries and the cuvettes. Although we are not in a position to reach a final explanation of this, we suggest that this could be related to the platelet anchoring and the container geometry (flat or cylindrical) which is translated throughout the sample, in accordance with previous results from nematic organization of non-delaminated clay particles [39]. Another factor that possibly could play a role here is the container material, for instance in our experiments the capillaries were made from borosilicate glass whereas the cuvettes are made from quartz, and these materials could possibly have different influence on the clay anchoring and packing density. Pre-aligning due to shear forces during filling may also play a role.
NaHec tactoids originally consist of N ~ 500 nanosheets, which are forced to separate while being forced into coplanar arrangement and at the same time align to the container during swelling. Also, we note that the size of the beam ensures that a large amount of the nematic domains are fully inside the scattering volume. Nanosheets originating from one tactoid lose correlation to each other to a certain degree as the domains swell. In consequence, the coherent correlation size (measure for averaged strictly coplanar stacked monolayers in the domain) determined by modeling the tactoids in the suspension as stack of coplanar oriented disks must decrease. Indeed, in the investigated concentration regime these correlation lengths are in the order of 200–500 nm, which corresponds to ca. 2 to 5 layers only and suggests pronounced local disorder in the melting regime. These results are summarized in Table S1 and in Fig. 7, which shows a representation of the nanosheet organization in the two geometries that we have investigated here. Figure 7 suggests correlation lengths in particle stacks that extend up to about 500 nm, with well-oriented stacks of particles extending over very much larger distances up to the millimeter range. This is illustrated by optical microscopy observations of accordion suspensions, see Fig. S9.
Sketch of nanosheet organization in two different types of confinement. The SAXS results suggest domain organizations as sketched for both investigated geometries, flat cuvettes and cylindrical capillaries, including both inter domain and coherent intra-domain lamellar organization. For both type of containers, a lamellar correlation length of approximately 200 to 500 nm is found from the fits (Fig. 5), whereas the azimuthal plots (Figs. 6, S7, and S8) suggest that the domain orientations are quite different for the two types of confinement. The illustration is out of proportion with respect to nanosheet diameter compared to container dimensions
The purpose of Fig. 7 is to illustrate the liquid crystalline organization in the two different geometries. In addition to sliding of platelets, we suggest a correlated lamellar liquid crystalline organization with disorder, due to nanosheets twisting, buckling, or wrinkling.
We suggest that this local disorder within long-range ordered nematic domains is directly related to the non-iridescent structural coloration observed in this system [15]. This is illustrated in Movie S1 in the supplementary information (rotating the capillary seen in Fig. 2b by 180° around its axis), which clearly demonstrates an angle independent behavior, despite the pronounced texture and the suggested wall-anchoring illustrated in Fig. 7. This also demonstrates that the structural coloration comes from Bragg interference and not from refraction, which would result in iridescence during such a rotation.
Conclusions
We have demonstrated that the geometry of the container used for suspensions of 2D nanosheets, NaHec in our case, directly affects the alignment of nanosheets and their effective d-spacing, as measured by SAXS. We find that the clay nanosheets form nematically ordered flat coherent subdomains. The lamellar intra-domain organization of nanosheets being forced in approximately coplanar arrangement by steric restrictions warrants interference with visible light. At the same time, the local disorder by tilting, translation, and undulation of flexible nanosheets limits the coherence length as observed with X-rays to a few monolayers. This study aids in understanding the factors that influence the internal organization in these nanosheet suspensions, and thus opens for the possibility of controlled color variation by systematic adjustment of the geometry that encapsulates the suspension and/or the characteristics of the individual nanoparticles.
In the present work, we used the same type of cuvettes that were previously used for analysis of structural colors. In the future, we plan to study suspensions in specially designed cuvettes, with both different geometries and different material types. In future works, magnetic or electric fields should be applied to assist orientation of nematic domains. Also, investigations of influence of flow, of confinement geometries as well as aqueous ionic strength should be performed. It could also be of interest to study these systems using scattering beams with a smaller or larger cross-sectional area in order to look for any structural ordering at different length scales.
Spatial and orientational distribution of high aspect ratio platelets in suspension is largely defined by filling conditions and the confinement of sample container. This knowledge enables the targeted design of hectorite-based templates showing structural color. Maybe even the degree of iridescence (higher for non-bending/non-undulating platelets) may be tuned. Our findings greatly enhance the understanding of the interplay between orientation in suspension, swelling behavior, and sample container geometry, thus providing a rationale for controlling the alignment in new materials/application, for instance in coating fabrication.
Data availability
No datasets were generated or analysed during the current study.
References
Bergaya F, Lagaly G, Beneke K (2006) Chapter 15 History of clay science: a young discipline. In: Bergaya F, Theng BKG, Lagaly G (eds) Developments in clay science. Handbook of clay science, vol 1. Elsevier, pp 1163–1181. https://doi.org/10.1016/S1572-4352(05)01043-3
Fossum JO (2020) Clay nanolayer encapsulation, evolving from origins of life to future technologies. Eur Phys J Spec Top 229(17):2863–2879. https://doi.org/10.1140/epjst/e2020-000131-1
Dudko V, Khoruzhenko O, Weiß S, Daab M, Loch P, Schwieger W, Breu J (2023) Repulsive osmotic delamination: 1D dissolution of 2D materials. Adv Mater Technol 8(3):2200553. https://doi.org/10.1002/admt.202200553
Cussler EL, Hughes SE, Ward WJ, Aris R (1988) Barrier membranes. J Membr Sci 38(2):161–174. https://doi.org/10.1016/S0376-7388(00)80877-7
Habel C, Tsurko ES, Timmins RL, Hutschreuther J, Kunz R, Schuchardt DD, Rosenfeldt S, Altstädt V, Breu J (2020) Lightweight ultra-high-barrier liners for helium and hydrogen. ACS Nano 14(6):7018–7024. https://doi.org/10.1021/acsnano.0c01633
Habel C, Schöttle M, Daab M, Eichstaedt NJ, Wagner D, Bakhshi H, Agarwal S, Horn MA, Breu J (2018) High-barrier, biodegradable food packaging. Macromol Mater Eng 303(10):1800333. https://doi.org/10.1002/mame.201800333
Doblhofer E, Schmid J, Rieß M, Daab M, Suntinger M, Habel C, Bargel H, Hugenschmidt C, Rosenfeldt S, Breu J, Scheibel T (2016) Structural insights into water-based spider silk protein–nanoclay composites with excellent gas and water vapor barrier properties. ACS Appl Mater Interfaces 8(38):25535–25543. https://doi.org/10.1021/acsami.6b08287
Tsurko ES, Feicht P, Nehm F, Ament K, Rosenfeldt S, Pietsch I, Roschmann K, Kalo H, Breu J (2017) Large scale self-assembly of smectic nanocomposite films by doctor blading versus spray coating: impact of crystal quality on barrier properties. Macromolecules 50(11):4344–4350. https://doi.org/10.1021/acs.macromol.7b00701
Zhu QL, Du C, Dai Y, Daab M, Matejdes M, Breu J, Hong W, Zheng Q, Wu ZL (2020) Light-steered locomotion of muscle-like hydrogel by self-coordinated shape change and friction modulation. Nat Commun 11(1):5166. https://doi.org/10.1038/s41467-020-18801-1
Zhu QL, Dai CF, Wagner D, Daab M, Hong W, Breu J, Zheng Q, Wu ZL (2020) Distributed electric field induces orientations of nanosheets to prepare hydrogels with elaborate ordered structures and programmed deformations. Adv Mater 32(47):2005567. https://doi.org/10.1002/adma.202005567
Zhu QL, Dai CF, Wagner D, Khoruzhenko O, Hong W, Breu J, Zheng Q, Wu ZL (2021) Patterned electrode assisted one-step fabrication of biomimetic morphing hydrogels with sophisticated anisotropic structures. Adv Sci 8(24):2102353. https://doi.org/10.1002/advs.202102353
Hunvik KWB, Loch P, Cavalcanti LP, Seljelid KK, Røren PM, Rudić S, Wallacher D, Kirch A, Knudsen KD, Rodrigues Miranda C, Breu J, Bordallo HN, Fossum JO (2020) CO2 capture by nickel hydroxide interstratified in the nanolayered space of a synthetic clay mineral. J Phys Chem C 124(48):26222–26231. https://doi.org/10.1021/acs.jpcc.0c07206
Hunvik KWB, Seljelid KK, Wallacher D, Kirch A, Cavalcanti LP, Loch P, Røren PM, Michels-Brito PH, Droppa-Jr R, Knudsen KD, Miranda CR, Breu J, Fossum JO (2023) Intercalation of CO2 selected by type of interlayer cation in dried synthetic hectorite. Langmuir 39(14):4895–4903. https://doi.org/10.1021/acs.langmuir.2c03093
Rieß M, Siegel R, Senker J, Breu J (2020) Diammonium-pillared MOPS with dynamic CO2 selectivity. Cell Rep Phys Sci 1(10):100210. https://doi.org/10.1016/j.xcrp.2020.100210
Michels-Brito PH, Dudko V, Wagner D, Markus P, Papastavrou G, Michels L, Breu J, Fossum JO (2022) Bright noniridescent structural coloration from clay mineral nanosheet suspensions. Sci Adv 8(4):eabl8147. https://doi.org/10.1126/sciadv.abl8147
Langmuir I (1938) The role of attractive and repulsive forces in the formation of tactoids, thixotropic gels, protein crystals and coacervates. J Chem Phys 6(12):873–896. https://doi.org/10.1063/1.1750183
Onsager L (1949) The effects of shape on the interaction of colloidal particles. Ann N Y Acad Sci 51(4):627–659. https://doi.org/10.1111/j.1749-6632.1949.tb27296.x
van der Kooij FM, Lekkerkerker HNW (1998) Formation of nematic liquid crystals in suspensions of hard colloidal platelets. J Phys Chem B 102(40):7829–7832. https://doi.org/10.1021/jp981534d
Bailey L, Lekkerkerker HNW, Maitland GC (2014) Rheology modification of montmorillonite dispersions by colloidal silica. Rheol Acta 53(5):373–384. https://doi.org/10.1007/s00397-014-0765-3
Bailey L, Lekkerkerker HNW, Maitland GC (2015) Smectite clay – inorganic nanoparticle mixed suspensions: phase behaviour and rheology. Soft Matter 11(2):222–236. https://doi.org/10.1039/C4SM01717J
Paineau E, Philippe AM, Antonova K, Bihannic I, Davidson P, Dozov I, Gabriel JCP, Impéror-Clerc M, Levitz P, Meneau F, Michot LJ (2013) Liquid–crystalline properties of aqueous suspensions of natural clay nanosheets. Liq Cryst Rev 1(2):110–126. https://doi.org/10.1080/21680396.2013.842130
Michot LJ, Bihannic I, Maddi S, Funari SS, Baravian C, Levitz P, Davidson P (2006) Liquid–crystalline aqueous clay suspensions. Proc Natl Acad Sci 103(44):16101–16104. https://doi.org/10.1073/pnas.0605201103
Ruzicka B, Zaccarelli EA (2011) Fresh look at the laponite phase diagram. Soft Matter 7(4):1268–1286. https://doi.org/10.1039/C0SM00590H
Angelini R, Zaccarelli E, de Melo Marques FA, Sztucki M, Fluerasu A, Ruocco G, Ruzicka B (2014) Glass–glass transition during aging of a colloidal clay. Nat Commun 5(1):4049. https://doi.org/10.1038/ncomms5049
Daab M, Eichstaedt NJ, Habel C, Rosenfeldt S, Kalo H, Schießling H, Förster S, Breu J (2018) Onset of osmotic swelling in highly charged clay minerals. Langmuir 34(28):8215–8222. https://doi.org/10.1021/acs.langmuir.8b00492
Marques FA, de Angelini M, Ruocco R, Ruzicka G (2017) Isotopic effect on the gel and glass formation of a charged colloidal clay: laponite. J Phys Chem B 121(17):4576–4582. https://doi.org/10.1021/acs.jpcb.6b12596
Michot LJ, Baravian C, Bihannic I, Maddi S, Moyne C, Duval JFL, Levitz P, Davidson P (2009) Sol–gel and isotropic/nematic transitions in aqueous suspensions of natural nontronite clay. Influence of particle anisotropy. 2. Gel structure and mechanical properties. Langmuir 25(1):127–139. https://doi.org/10.1021/la801894a
Michot LJ, Bihannic I, Porsch K, Maddi S, Baravian C, Mougel J, Levitz P (2004) Phase diagrams of Wyoming Na-montmorillonite clay. Influence of particle anisotropy. Langmuir 20(25):10829–10837. https://doi.org/10.1021/la0489108
Shalkevich A, Stradner A, Bhat SK, Muller F, Schurtenberger P, Cluster (2007) Glass, and gel formation and viscoelastic phase separation in aqueous clay suspensions. Langmuir 23(7):3570–3580. https://doi.org/10.1021/la062996i
Paineau E, Bihannic I, Baravian C, Philippe A-M, Davidson P, Levitz P, Funari SS, Rochas C, Michot LJ (2011) Aqueous suspensions of natural swelling clay minerals. 1. Structure and electrostatic interactions. Langmuir 27(9):5562–5573. https://doi.org/10.1021/la2001255
Mourad Mcd, Wijnhoven Jegj, van ’t Zand Dd, van der Beek D, Lekkerkerker Hnw (2006) Gelation versus liquid crystal phase transitions in suspensions of plate-like particles. Philos Trans R Soc Math Phys Eng Sci 364(1847):2807–2816. https://doi.org/10.1098/rsta.2006.1856
Davidson P, Gabriel J-CP (2005) Mineral liquid crystals. Curr Opin Colloid Interface Sci 9(6):377–383. https://doi.org/10.1016/j.cocis.2004.12.001
Hansen EL, Hemmen H, Fonseca DM, Coutant C, Knudsen KD, Plivelic TS, Bonn D, Fossum JO (2012) Swelling transition of a clay induced by heating. Sci Rep 2(1):618. https://doi.org/10.1038/srep00618
Fossum JO, Gudding E, Fonseca DdM, Meheust Y, DiMasi E, Gog T, Venkataraman C (2005) Observations of orientational ordering in aqueous suspensions of a nano-layered silicate. Energy 30(6):873–883. https://doi.org/10.1016/j.energy.2004.04.008
DiMasi E, Fossum JO, Gog T, Venkataraman C (2001) Orientational order in gravity dispersed clay colloids: a synchrotron X-ray scattering study of Na fluorohectorite suspensions. Phys Rev E 64(6):061704. https://doi.org/10.1103/PhysRevE.64.061704
Fonseca DM, Méheust Y, Fossum JO, Knudsen KD, Parmar KPS (2009) Phase diagram of polydisperse Na-fluorohectorite–water suspensions: a synchrotron small-angle X-ray scattering study. Phys Rev E 79(2):021402. https://doi.org/10.1103/PhysRevE.79.021402
Hemmen H, Ringdal NI, De Azevedo EN, Engelsberg M, Hansen EL, Méheust Y, Fossum JO, Knudsen KD (2009) The isotropic−nematic interface in suspensions of Na−fluorohectorite synthetic clay. Langmuir 25(21):12507–12515. https://doi.org/10.1021/la901784k
Daab M, Rosenfeldt S, Kalo H, Stöter M, Bojer B, Siegel R, Förster S, Senker J, Breu J (2017) Two-step delamination of highly charged, vermiculite-like layered silicates via ordered heterostructures. Langmuir 33(19):4816–4822. https://doi.org/10.1021/acs.langmuir.7b01008
de Azevedo EN, Engelsberg M, Fossum JO, de Souza RE (2007) Anisotropic water diffusion in nematic self-assemblies of clay nanoplatelets suspended in water. Langmuir 23(9):5100–5105. https://doi.org/10.1021/la0632629
Ringdal NI, Fonseca DM, Hansen EL, Hemmen H, Fossum JO (2010) Nematic textures in colloidal dispersions of Na-fluorohectorite synthetic clay. Phys Rev E 81(4):041702. https://doi.org/10.1103/PhysRevE.81.041702
Stöter M, Kunz DA, Schmidt M, Hirsemann D, Kalo H, Putz B, Senker J, Breu J (2013) Nanoplatelets of sodium hectorite showing aspect ratios of ≈ 20 000 and superior purity. Langmuir 29(4):1280–1285. https://doi.org/10.1021/la304453h
Rosenfeldt S, Stöter M, Schlenk M, Martin T, Albuquerque RQ, Förster S, Breu J (2016) In-depth insights into the key steps of delamination of charged 2D nanomaterials. Langmuir 32(41):10582–10588. https://doi.org/10.1021/acs.langmuir.6b02206
El Rifaii K, Wensink HH, Dozov I, Bizien T, Michot LJ, Gabriel J-CP, Breu J, Davidson P (2022) Do aqueous suspensions of smectite clays form a smectic liquid-crystalline phase? Langmuir 38(48):14563–14573. https://doi.org/10.1021/acs.langmuir.2c01821
Kalo H, Möller MW, Ziadeh M, Dolejš D, Breu J (2010) Large scale melt synthesis in an open crucible of Na-fluorohectorite with superb charge homogeneity and particle size. Appl Clay Sci 48(1):39–45. https://doi.org/10.1016/j.clay.2009.11.014
Förster S, Apostol L, Bras W (2010) It scatter: software for the analysis of nano- and mesoscale small-angle scattering. J Appl Crystallogr 43(3):639–646. https://doi.org/10.1107/S0021889810008289
Doucet M, Cho JH, Alina G, Attala Z, Bakker J, Bouwman W, Butler P, Campbell K, Cooper-Benun T, Durniak C, Forster L, Gonzalez M, Heenan R, Jackson A, King S, Kienzle P, Krzywon J, Murphy R, Nielsen T, O’Driscoll L, Potrzebowski W, Prescott S, Ferraz Leal R, Rozyczko P, Snow T, Washington A (2021) SasView version 5.0.4. Zenodo. https://doi.org/10.5281/zenodo.4467703
Stöter M, Gödrich S, Feicht P, Rosenfeldt S, Thurn H, Neubauer JW, Seuss M, Lindner P, Kalo H, Möller M, Fery A, Förster S, Papastavrou G, Breu J (2016) Controlled exfoliation of layered silicate heterostructures into bilayers and their conversion into giant janus platelets. Angew Chem 128(26):7524–7528. https://doi.org/10.1002/ange.201601611
Lindemann FA (1910) The calculation of molecular vibration frequencies. Z Phys 11:609–615
Acknowledgements
The authors thank Leide Cavalcanti and Adrian Rennie for fruitful discussions and comments. We are indebted to Florian Puchtler for synthesizing the hectorite, and we also acknowledge the Dutch-Belgian beamline (DUBBLE) at ESRF for the beam time used for the SAXS experiments and Martin Rosenthal for invaluable support both before and during the experiments. We acknowledge MAX IV Laboratory for time on Beamline CoSAXS under Proposals 20210657 and 20220537 for preliminary experiments. Research conducted at MAX IV, a Swedish national user facility, is supported by the Swedish Research Council under contract 2018–07152, the Swedish Governmental Agency for Innovation Systems under contract 2018–04969, and Formas under contract 2019–02496. Furthermore, this work benefited from the use of the SasView application, originally developed under NSF award DMR-0520547. SasView contains code developed with funding from the European Union’s Horizon 2020 research and innovation programme under the SINE2020 project, grant agreement No 654000.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital) This work was supported by the Research Council of Norway (RCN) under the PETROMAKS2 program, project number 280643 (O.T.N., K.K.S., K.D.K., J.O.F.), under the RCN FRIPRO program, project number 315135 (P.H.M.B., J.O.F.), under the EU Horizon 2020 MSCA-ITN-2020, project number 956248 (B.T.C., R.P.R., K.D.K., J.O.F.), NTNU (A.A.), and German Science Foundation (project number 492723217 (CRC 1585) (J.B., S.R.).
Author information
Authors and Affiliations
Contributions
O.T.N.: did nearly all the data analysis, wrote the first draft of the text, edited and completed the final manuscript based on discussions with coauthors, made all figures and plots as well as some of the acquisition of data. S.R.: assisted with analysis and interpretation of data, as well as major contributions to final manuscript and its organization. P.H.M.B.: prepared some of the samples, assisted with preliminary experiments data acquisition, took part in discussions. K.K.S.: data acquisition and planning of some of the experiments. A.A.: assisted with data acquisition. B.T.C.: assisted with data acquisition. R.P.R.: assisted with data acquisition. T.S.P.: assisted with data acquisition for preliminary experiments and with the design of the work, contributed with discussions. L.M.: assisted with interpretation of data and figure design ideas, took part in discussions. J.B.: major assistance with interpretation and discussion of data as well as major contributions to final manuscript and its organization. K.D.K.: assisted with interpretation of data and design of the work, assisted with analysis and interpretation of data as well as contribution to final manuscript and its organization. J.O.F.: initiated and conceptualized the work, supervised some of the data acquisition, provided major assistance with interpretation and discussion of data, as well as major contributions to final manuscript and its organization.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Dedicated to Prof. Dr. Stephan Förster on the occasion of his 60th birthday.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 4704 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trigueiro Neto, O., Rosenfeldt, S., Michels-Brito, P.H. et al. Liquid crystalline structuring in dilute suspensions of high aspect ratio clay nanosheets. Colloid Polym Sci (2024). https://doi.org/10.1007/s00396-024-05268-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00396-024-05268-5