Abstract
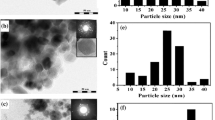
The generation of metal nanostructures by radiation-induced reduction of copper ions in aqueous dispersions of macromolecular complexes of poly(acrylic acid) and polyvinylimidazolе has been studied in the pH range of 2.3–4.3. It was shown that an effective coordination number of Cu2+ in the complex with polymer units decreased at lower pH, which resulted in dramatic increase of the nanoparticle formation rate. As demonstrated by transmission electron microscopy, using the poly(acrylic acid)–polyvinylimidazolе–Cu2+ complexes as precursors allows one to control the nanoparticle size and promotes assembling of spatially ordered supramolecular structures. As revealed by dynamic light scattering, decreasing the medium pH leads to ripening of the poly(acrylic acid)–polyvinylimidazolе–Cu2+ particles. The obtained results provide an evidence for strong effect of the pH value on the spatial organization of nanoparticles in irradiated suspensions. In the case of less acidic medium (at pH 3), assembling of nanoparticles occurs in flabby interpolymer particles, whereas at lower pH the metal nanoparticles are produced in the templates of the perfect macromolecular nanostructures.








Similar content being viewed by others
References
Bronstein LM, Sidorov SN, Valetsky PM (2004) Nanostructured polymeric systems as nanoreactors for nanoparticle formation. Russ Chem Rev 73:501–515. https://doi.org/10.1070/RC2004v073n05ABEH000782
Lu Y, Ballauff M (2011) Thermosensitive core-shell nicrogels: from colloidal nodel systems to nanoreactors. Prog Polym Sci 36:767–792. https://doi.org/10.1557/PROC-1234-QQ08-04
Pergushov DV, Zezin AA, Zezin AB, Müller AHE (2014) Advanced functional structures based on interpolyelectrolyte complexes. Adv Polym Sci 25:173–225. https://doi.org/10.1007/12_2012_182
Broz P (2010) Polymer based nanostructures: medical applications. RSC Publishing, Cambridge
Wang Y, Asefa T (2009) Poly(allylamine)-stabilized colloidal copper nanoparticles: synthesis, morphology, and their surface-enhanced Raman scattering properties. Langmuir 26:7469–7474. https://doi.org/10.1021/la904199f
Lu YZ, Wei WT, Chen W (2012) Copper nanoclusters: synthesis, characterization and properties. Chin Sci Bull 57:41–47. https://doi.org/10.1007/s11434-011-4896-y
Vukojevic S, Trapp O, Grunwaldt J, Kiener C, Schüth F (2005) Quasi-homogeneous methanol synthesis over highly active copper nanoparticles. Angew Chem Int Ed 44:7978–7981. https://doi.org/10.1002/anie.200503169
Khatouri J, Mostafavi M, Amblard J, Belloni J (1992) Radiation-induced copper aggregates and oligomers. Chem Phys Lett 191:351–356. https://doi.org/10.1016/0009-2614(92)85313-Y
Litmanovich OE, Ostaeva GY, Tatarinov VS, Bogdanov AG, Papisov IM (2010) Effect of complexation of poly(acrylic acid) with Cu2+ ions on the size of copper nanoparticles prepared via reduction in aqueous solutions. Polym Sci B 52:397–407. https://doi.org/10.1134/S1560090410070043
Zezin AA, Feldman VI, Abramchuk SS, Danelyan GV, Dyo VV, Plamper FA, Müller AHE, Pergushov DV (2015) Efficient size control of copper nanoparticles generated in irradiated aqueous solutions of star-shaped polyelectrolyte containers. Phys Chem Chem Phys 17:11490–11498. https://doi.org/10.1039/c5cp00269a
Ostaeva GY, Papisov IM, Selishcheva ED, Arbuzov DE (2010) Mutual enhancement of the complexing properties of components in tertiary systems containing copper nanoparticles, poly(acrylic acid), and poly(ethylene glycol). Polym Sci B 52:86–90. https://doi.org/10.1134/S1560090410010148
Ostaeva GY, Papisov IM, Arbuzov DE, Papisova AI (2013) Specific features of nonstoichiometric interpolymer complexes of poly(acrylic acid) and poly(ethylene glycol) as protectors of copper nanoparticles in aqueous sols. Polym Sci A 55:253–257. https://doi.org/10.1134/S0965545X13040044
Bakar A, Dyo V, Zezin AA, Abramchuk SS, Güven O, Feldman VI (2012) Spatial organization of a metal–polymer nanocomposite obtained by the radiation-induced reduction of copper ions in the poly(allylamine)–poly(acrylic acid)–Cu2+ system. Mendeleev Commun 22:211–212. https://doi.org/10.1016/j.mencom.2012.06.014
Bakar A, Güven O, Zezin AA, Feldman VI (2014) Controlling the size and distribution of copper nanoparticles in double and triple polymer metal complexes by X-ray irradiation. Radiat Phys Chem 94:62–65. https://doi.org/10.1016/j.radphyschem.2013.07.006
Belloni J (2006) Nucleation, growth and properties of nanoclusters studied by radiation chemistry: application to catalysis. Catal Today 113:141–156. https://doi.org/10.1016/j.cattod.2005.11.082
Ershov BG (1997) Metal ions in unusual and unstable oxidation states in aqueous solutions: preparation and properties. Russ Chem Rev 66:93–105. https://doi.org/10.1070/RC1997v066n02ABEH000264
Ershov BG (1994) Colloidal copper in aqueous solutions: radiation-chemical reduction, mechanism of formation, and properties. Russ Chem Bull 43:16–21. https://doi.org/10.1007/BF00699128
Joshi SS, Patil SF, Lyer V, Mahumuni S (1998) Radiation induced synthesis and characterization of copper nanoparticles. Nanostruct Mater 10:1135–1144. https://doi.org/10.1016/S0965-9773(98)00153-6
Pekel N, Güven O (1999) Investigation of complex formation between pol(N-vinyl imidazole) and various metal ions using the molar ratio method. Colloid Polym Sci 277:570–573. https://doi.org/10.1007/s003960050426
Hanawalt JD, Rinn HW, Frevel LK (1938) Chemical analysis by X-ray diffraction. Ind Eng Chem Anal Ed 10:457–512. https://doi.org/10.1017/s0885715600011490
Zezin AA, Feldman VI, Zezina EA, Belopushkin SI, Tsybina EV, Abramchuk SS, Zezin SB (2011) The formation of metal nanoparticles in polyacrylic acid-polyethyleneimine complex upon reduction of copper(II) ions using X-ray irradiation. High Energy Chem 45:99–103. https://doi.org/10.1134/S0018143911020147
Zezin AA, Feldman VI, Dudnikov AV, Zezin SB, Abramchuk SS, Belopushkin SI (2009) Reduction of copper(II) ions in polyacrylic acid-polyethyleneimine complexes using X-ray radiation. High Energy Chem 43:100–104. https://doi.org/10.1134/S0018143909020064
Murray RW (2008) Nanoelectrochemistry: metal nanoparticles, nanoelectrodes, and nanopores. Chem Rev 108:2688–2720. https://doi.org/10.1021/cr068077e
Annenkov VV, Danilovtseva EN, Saraev VV, Mikhaleva AI (2003) Complexation of copper (II) ions with imidazole-carboxylic polymeric systems. J Polym Sci Part A: Polym Chem 41:2256–2263. https://doi.org/10.1002/pola.10769
Demchenko VL, Shtompel VI (2014) Structuring, morphology, and thermomechanical properties of nanocomposites formed from ternary polyelectrolyte-metal complexes based on pectin, polyethyleneimine and CuSO4. Polymer Sci B 56:927–934. https://doi.org/10.1134/S1560090414060049
Zezin AA, Feldman VI, Shmakova NA, Valueva SP, Ivanchenko VK, Nikanorova NI (2007) The peculiarities of formation of the metal nanoparticles in irradiated polymer metal complexes. Nucl Inst Methods Phys Res B 265:334–338. https://doi.org/10.1016/j.nimb.2007.08.068
Zezin AA, Klimov DI, Zezina EA, Mkrtchyan KV, Feldman VI (2019) Controlled radiation-chemical synthesis of metal polymer nanocomposites in the films of interpolyelectrolyte complexes: principles, prospects and implications. Radiat Phys Chem In press. https://doi.org/10.1016/j.radphyschem.2018.11.030
Zezina EA, Emel’yanov AI, Pozdnyakov AS, Prozorova GF, Abramchuk SS, Feldman VI, Zezin AA (2019) Radiation-induced synthesis of copper nanostructures in the films of interpolymer complexes. Radiat Phys Chem 158:115–121. https://doi.org/10.1016/j.radphyschem.2019.01.019
Lampre I, Pernot P, Mostafavi M (2000) Spectral properties and redox potentials of silver atoms complexed by chloride ions in aqueous solution. J Phys Chem B 104:6233–6239. https://doi.org/10.1021/jp000544n
Rémita S, Archirel P, Mostafavi M (1995) Evaluation of the redox potential of Ag1I(CN)2−/Ag10(CN)22− in aqueous solution. J Phys Chem 99:13198–13202. https://doi.org/10.1021/j100035a025
Rémita S, Mostafavi M, Delcourt MO (1996) EDTA and CN− complexing effect on the kinetics, spectral properties, and redox properties of Ag10 and Ag2+ in aqueous solution. J Phys Chem 100:10187–10193. https://doi.org/10.1021/jp960176g
Texier I, Rémita S, Archirel P, Mostafavi M (1996) Reduction of AgI1(NH3)2+ to Ag01 (NH3)2 in solution. Redox potential and spectral study. J Phys Chem 100:12472–12476. https://doi.org/10.1021/jp9535654
Annenkov VV, Mazyar NL, Kruglova VA, Ananiev SM (2001) Equilibria in solutions of complexes of poly(acrylic acids) and poly(N-vinylazoles). J Mol Liq 91:109–114. https://doi.org/10.1016/S0167-7322(01)00152-0
Mazyar NL, Annenkov VV, Kruglova VA, Toryashinova D-SD, Danilovtseva EN (1999) Interaction of poly(acrylic acid) with polyvinyl(1-vinylimidazole). Polym Sci A 41:246–251
Annenkov VV (2001) The reaction of complex formation of polivinylazoles. Thesis, Irkutsk, Russia
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was supported by the Russian Foundation for Basic Research, project no: 18-03-00608 and by the Scientific and Technological Council of Turkey (TUBITAK), project no: 210T077.
Electronic supplementary material
Additional data for DLS, UV-VIS spectroscopy, TEM and turbidimetric titration
ESM 1
(DOCX 252 kb)
Rights and permissions
About this article
Cite this article
Dağaş, D.E., Danelyan, G.V., Ghaffarlou, M. et al. Generation of spatially ordered metal–polymer nanostructures in the irradiated dispersions of poly(acrylic acid)–poly(vinylimidazole)–Cu2+ complexes. Colloid Polym Sci 298, 193–202 (2020). https://doi.org/10.1007/s00396-019-04592-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-019-04592-5