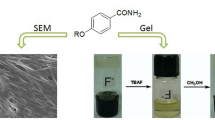
Abstract
Organogels composed of 40% hydrolyzed poly(vinyl acetate) (40PVAc) and three arylboronic acids have been formed and characterized in several liquids to gain insights into the role of crosslinker length and structure on the properties of the gels. Data from 1H NMR, steady-state and time-resolved fluorescence, and rheology have been employed to correlate the degrees of crosslinking and the bulk physical properties of the gels. Benzene-1,4-diboronic acid (1,4-BDBA) and 4,4′-biphenyldiboronic acid (bPDBA) formed gels with 40PVAc that were stable for longer periods than those with benzene-1,3-diboronic acid. Surprisingly, the steady-state and time-resolved fluorescence properties of 1,4-BDBA or bPDBA were affected very little in solution upon addition of two model diols (1,3-propanediol and 1,2-ethanediol) or even 40PVAc. Furthermore, 40PVAc appeared to form crosslinks more efficiently, resulting in stiffer gels, with bPDBA than with 1,4-BDBA. We attribute these trends to the greater length, flexibility, and linearity of bPBPA. Overall, the results reported here demonstrate that minor structural changes in the crosslinker can alter significantly many of the bulk properties of two-component organogel networks. They also provide a clear ‘blueprint’ for making gels with specific desired properties for a range of applications that require virtually water-free systems.









Similar content being viewed by others
References
Hall DG (2011) Boronic acids. Wiley-VCH Verlag GmbH & Co KGaA, Weinheim
Yan J, Springsteen G, Deeter S, Wang B (2004) The relationship among pK a, pH, and binding constants in the interactions between boronic acids and diols - it is not as simple as it appears. Tetrahedron 60:11205–11209. https://doi.org/10.1016/j.tet.2004.08.051
Adamczyk-Woźniak A, Jakubczyk M, Jankowski P, Sporzyński A, Urbański PM (2013) Influence of the diol structure on the Lewis acidity of phenylboronates. J Phys Org Chem 26:415–419. https://doi.org/10.1002/poc.3102
Guan Y, Zhang Y (2013) Boronic acid-containing hydrogels: synthesis and their applications. Chem Soc Rev 42:8106–8121. https://doi.org/10.1039/c3cs60152h
Brooks WLA, Sumerlin BS (2016) Synthesis and applications of Boronic acid-containing polymers: from materials to medicine. Chem Rev 116:1375–1397. https://doi.org/10.1021/acs.chemrev.5b00300
Kikuchi A, Suzuki K, Okabayashi O, Hoshino H, Kataoka K, Sakurai Y, Okano T (1996) Glucose-sensing electrode coated with polymer complex gel containing Phenylboronic acid. Anal Chem 68:823–828. https://doi.org/10.1021/ac950748d
Piest M, Zhang X, Trinidad J, Engbersen JFJ (2011) pH-responsive, dynamically restructuring hydrogels formed by reversible crosslinking of PVA with phenylboronic acid functionalised PPO–PEO–PPO spacers (Jeffamines®). Soft Matter 7:11111–11118. https://doi.org/10.1039/c1sm06230a
Meng H, Zheng J, Wen XF, Cai Z, Zhang J, Chen T (2015) PH- and sugar-induced shape memory hydrogel based on reversible phenylboronic acid-diol ester bonds. Macromol Rapid Commun 36:533–537. https://doi.org/10.1002/marc.201400648
Ding Z, Guan Y, Zhang Y, Zhu XX (2009) Layer-by-layer multilayer films linked with reversible boronate ester bonds with glucose-sensitivity under physiological conditions. Soft Matter 5:2302–2309. https://doi.org/10.1039/b901910c
Zhang X, Guan Y, Zhang Y (2012) Ultrathin hydrogel films for rapid optical biosensing. Biomacromolecules 13:92–97. https://doi.org/10.1021/bm2012696
Zhao YN, Yuan Q, Li C, Guan Y, Zhang Y (2015) Dynamic layer-by-layer films: a platform for zero-order release. Biomacromolecules 16:2032–2039. https://doi.org/10.1021/acs.biomac.5b00438
Zhang D, Yu G, Long Z, Yang G, Wang B (2016) Controllable layer-by-layer assembly of PVA and phenylboronic acid-derivatized chitosan. Carbohydr Polym 140:228–232. https://doi.org/10.1016/j.carbpol.2015.12.032
Kotsuchibashi Y, Ebara M, Sato T, Wang Y, Rajender R, Hall DG, Narain R, Aoyagi T (2015) Spatiotemporal control of synergistic gel disintegration consisting of boroxole- and glyco-based polymers via photoinduced proton transfer. J Phys Chem B 119:2323–2329. https://doi.org/10.1021/jp506478p
Kataoka K, Miyazaki H, Bunya M, Okano T, Sakurai Y (1998) Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on-off regulation of insulin release. J Am Chem Soc 120:12694–12695. https://doi.org/10.1021/ja982975d
Shiino D, Murata Y, Kataoka K, Koyama Y, Yokoyama M, Okano T, Sakurai Y (1994) Preparation and characterization of a glucose-responsive insulin-releasing polymer device. Biomaterials 15:121–128. https://doi.org/10.1016/0142-9612(94)90261-5
Ge H, Ding Y, Ma C, Zhang G (2006) Temperature-controlled release of diols from N-isopropylacrylamide-co-acrylamidophenylboronic acid microgels. J Phys Chem B 110:20635–20639. https://doi.org/10.1021/jp060914t
Du X, Jiang G, Li L et al (2015) Photo-induced synthesis glucose-responsive carriers for controlled release of insulin in vitro. Colloid Polym Sci 293:2129–2135. https://doi.org/10.1007/s00396-015-3625-5
Cash JJ, Kubo T, Bapat AP, Sumerlin BS (2015) Room-temperature self-healing polymers based on dynamic-covalent boronic esters. Macromolecules 48:2098–2106. https://doi.org/10.1021/acs.macromol.5b00210
Cromwell OR, Chung J, Guan Z (2015) Malleable and self-healing covalent polymer networks through tunable dynamic Boronic Ester bonds. J Am Chem Soc 137:6492–6495
Deng CC, Brooks WLA, Abboud KA, Sumerlin BS (2015) Boronic acid-based hydrogels undergo self-healing at neutral and acidic pH. ACS Macro Lett 4:220–224. https://doi.org/10.1021/acsmacrolett.5b00018
Nishiyabu R, Kobayashi H, Kubo Y (2012) Dansyl-containing boronate hydrogel film as fluorescent chemosensor of copper ions in water. RSC Adv 2:6555–6561. https://doi.org/10.1039/c2ra20516e
Parris MD, MacKay BA, Rathke JW et al (2008) Influence of Pressure on Boron Cross-Linked Polymer Gels. Macromol (Washington, DC, United States) 41:8181–8186. https://doi.org/10.1021/ma801187q
Nunes MAP, Martins S, Rosa ME, Gois PMP, Fernandes PCB, Ribeiro MHL (2015) Improved thermostable polyvinyl alcohol electrospun nanofibers with entangled naringinase used in a novel mini-packed bed reactor. Bioresour Technol 213:208–215. https://doi.org/10.1016/j.biortech.2016.03.058
Nunes MAP, Gois PMP, Rosa ME, Martins S, Fernandes PCB, Ribeiro MHL (2016) Boronic acids as efficient cross linkers for PVA: synthesis and application of tunable hollow microspheres in biocatalysis. Tetrahedron 72:7293–7305. https://doi.org/10.1016/j.tet.2016.02.017
Duncan T, Berrie B, Weiss R (2017) Soft, Peelable Organogels from partially hydrolyzed poly(vinyl acetate) and Benzene-1,4-diboronic acid: applications to clean works of art. ACS Appl Mater Interfaces 9:28069–28078
Acknowledgements
We thank the US National Science Foundation (Grant CHE-1502856) for supporting part of this research. TTD thanks the Achievement Rewards for College Scientists/Metro Washington Chapter for a scholar award. The Kuraray Co., Ltd. is thanked for donating the xPVAc samples employed in this research. We are grateful to Ting-An Chen for collecting the time-resolved fluorescence decays.
Dedication
We dedicate this article to Dr. Eduardo Lissi Gervaso who became ‘Profesor Emérito’ of the Universidad de Santiago de Chile on 29 November 2017. He has been a wonderful inspiration to many in his roles as a superb scientist and a principled human being. Congratulations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 1015 kb)
Rights and permissions
About this article
Cite this article
Duncan, T.T., Weiss, R.G. Influence of length and structure of aryl boronic acid crosslinkers on organogels with partially hydrolyzed poly(vinyl acetate). Colloid Polym Sci 296, 1047–1056 (2018). https://doi.org/10.1007/s00396-018-4326-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4326-7