Abstract
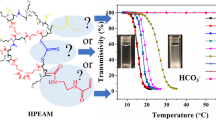
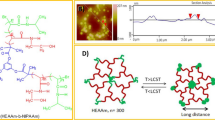
A hyperbranched copolymer, hyperbranched-g-(N-isopropylacrylamide-co-itaconamic acid) (“hyperbranch-g-(NIPAAm-co-IAM)”), serving as a pH- and thermo-responsive material, was synthesized via atomic transfer radical polymerization (ATRP) and characterized in this study. A macroinitiator was prepared from a commercially available hyperbranched polymer, “hyperbranched bis-(methylol)propionic acid (bis-MPA) polyester-16-hydroxyl, generation 2” (hyperbranch-OH) and used as a core structure of the hyperbranched copolymer. The hyperbranch-OH has 16 hydroxyl groups; among them, 12 arms were derived from substitution of the hydroxyl groups with poly(NIPAAm-co-IAM) by ATRP. The polydispersity index (PDI) of the newly synthesized hyperbranched copolymer can be controlled to be less than 1.4. The hyperbranched copolymer shows more significant size change as the environmental pH value changes, compared to the linear random poly(NIPAAm-co-IAM). The particle size decreases with temperature but increases with the pH value. As the molar fraction of IAM in the hyperbranched copolymer increases, the lower critical solution temperature (LCST) increases. Hyperbranch-g-(NIPAAm-co-IAM) with a molar ratio of NIPAAm/IAM equaling to 3:1 shows a LCST of the aqueous solution close to the body temperature, indicating potential use in biomedical applications such as drug release.










Similar content being viewed by others
References
Flory PJ (1952) Principles of polymer chemistry, chapter 9. Cornell University, Press, Ithaca, NY
Tomalia DA, Baker H, Dewald JR, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P (1985) A new class of polymers: starburst-dendritic macromolecules. Polymer J 17:117–132
Newkome GR, Moorefield CN, Baker GR (1992) Building blocks for dendritic macromolecules. Aldrichimica Acta 25:31–38
Dvornic PR, Tomalia DA (1996) Recent advances in dendritic polymers. Curr Opin Colloid Interface Sci 1(2):221–235
Medina SH, El-Sayed MEH (2009) Dendrimers as carriers for delivery of chemotherapeutic agents. Chem Rev 109:3141–3157
Fréchet JMJ (1994) Functional polymers and dendrimers: reactivity, molecular architecture, and interfacial energy. Science 263:1710–1715
Fréchet JMJ, Hawker CJ (1995) Hyperbranched polyphenylene and hyperbranched polyesters: new soluble, three-dimensional, reactive polymers. Reactive Funct Polymers 26:127–136
Stancu IC (2010) Gelatin hydrogels with PAMAM nanostructured surface and high density surface-localized amino groups. Reactive Funct Polymers 70:314–324
Menjoge AR, Kannan RM, Tomalia DA (2010) Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today 15(5/6):171–185
Malmström E, Hult A (1996) Kinetics of formation of hyperbranched polyesters based on 2,2-bis(methylol)propionic acid. Macromolecules 29:1222–1228
Zheng Y, Quinn J, Yan H, Hu Y, Lu S, Facchetti A. Dielectric materials and methods of preparation and use thereof, US patent application No. 20110175089
Zhou G, Zhao Y, Hu J, Shen L, Liu W, Yang X (2013) A new drug-loading technique with high efficaciency and sustained-releasing ability via the Pickering emulsion interfacial assembly of temperature/pH-sensitive nanogels. Reactive Funct Polymers 73:1537–1543
Rwei SP, Way TF, Chang SM, Chiang WY, Lien YY (2015) Thermo- and pH- responsive copolymers: poly(N-isopropylacrylamide-co-IAM) copolymers, J Appl Polym Sci (in print)
Rwei SP, Chuang YY, Way TF, Chiang WY, Hsu SP (2015) Preparation of thermo- and pH- responsive star copolymers via ATRP and used in drug release application. Colloid Polym Sci 293:493–503
Aloorkar NH, Kulkarmi AS, Patil RA, Ingale DJ (2012) Star polymers: an overview. Inter J Pharm Sci Nanotech 5:1675–1684
Bai Y, Wei J, Yang L, He C, Lu X (2012) Temperature and pH dual-responsive behavior of polyhedral oligomeric silsesquioxane-based star-block copolymer with poly(acrylic acid-block-N-isopropylacrylamide) as arms. Colloid Polym Sci 290(6):507–515
Hadjichristidis N, Pitsikalis M, Iatrou H, Driva P, Sakellariou G, Chatzichristidi M (2012) Polymers with star-related structures: synthesis, properties, and applications, In: Matyjaszewski K and Möller M (eds) Polymer science: a comprehensive reference. Elsevier, Amsterdam, 6.03:29
Li P, Xu R, Wang W, Li X, Xu Z, Yeung K, Chu P (2013) Thermosensitive poly(N-isopropylacrylamide-co-glycidyl methacrylate) microgels for controlled drug release. Colloid Surf B Biointerfaces 101:251–25
Abdullah-Al-Nahain LKS, Mosaiab T, Park SY (2013) pH and thermo-responsive poly(N-isopropylacrylamide) copolymer grafted to poly(ethylene glycol). J Appl Polym Sci 10:168–174
Lee W-F, Cheng T-S (2013) Effect of monomer composition on the properties of biodegradable poly(NIPAAm-AA-PCLdA) copolymeric hydrogels. J Appl Polym Sci 10:230–238
Erbill C, Yildiz Y, Uyanik N (2009) N-isopropylacrylamide/monoalkyl itaconate copolymers and N-isopropylacrylamide/itaconic acid/dimethyl itaconate terpolymers. Polym Adv Technol 20:926–933
Kato M, Kamigaito M, Sawamoto M (1995) Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine) ruthenium(II)/methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization. Macromolecules 28(5):1721–1723
Wang JS, Matyjaszewski K (1995) Controlled/“living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J Am Chem Soc 117(20):5614–5615
Patten TE, Matyjaszewski K (1998) Atom transfer radical polymerization and the synthesis of polymeric materials. Adv Mater 10(12):901–915
Acknowledgments
The authors would like to thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under Contract No. NSC 102-2218-E-027-015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rwei, SP., Shu, KT., Way, TF. et al. Synthesis and characterization of hyperbranched copolymers hyper-g-(NIPAAm-co-IAM) via ATRP. Colloid Polym Sci 294, 291–301 (2016). https://doi.org/10.1007/s00396-015-3775-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3775-5