Abstract
In this study, we report on a label-free biosensor for the detection of acetylcholine (ACh) and an acetylcholinesterase (AChE) inhibitor, utilizing liquid crystals (LCs) on a polymeric surface with periodic nanostructures. The polymeric nanostructures, which hold sinusoidal anisotropic patterns, were fabricated by a sequential process of poly(dimethylsiloxane) buckling and replication of these patterns on a poly(urethane acrylate) surface where a film of gold was deposited. AChE was then covalently immobilized onto the gold surface. Optical images of nematic 4-cyano-4′-pentylbiphenyl (5CB) revealed that it aligned parallel to the plane of the enzyme-decorated surface. However, AChE-mediated hydrolysis of ACh resulted in a plume of choline, acetate, and unreacted ACh, which in turn produced a distinctive optical transition of 5CB (from uniform to random) by masking the anisotropic surface topography. The hydrolysis of ACh was inhibited in the presence of a carbamate insecticide (AChE inhibitor), which prevented the orientational transition of 5CB by decreasing the enzymatic activity of AChE. These results suggest that this LC-based enzymatic sensor is highly sensitive to ACh and the AChE inhibitor, with a detection limit of 10 and 1 nM, respectively. This study demonstrates that polymeric surfaces with continuous wavy features can be used as LC-based biosensors to amplify the existence of ACh and an AChE inhibitor without labeling or an additional amplification strategy.

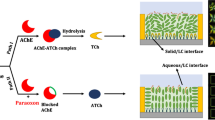
Graphical abstract for the LC-based label-free detection of ACh on a nanostructured polymeric surface






Similar content being viewed by others
References
Stanfield CL (2009) Principles of human physiology, 4th edn. Pearson Education Inc, San Francisco
The National Collaborating Centre for Chronic Conditions (2006) Parkinson’s disease. Royal College of Physicians, London
Francis PT, Palmer AM, Snape M, Wilcock GK (1999) The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 66:137–147. doi:10.1136/jnnp.66.2.137
Mehta M, Adem A, Sabbagh M (2012) New acetylcholinesterase inhibitors for Alzheimer’s disease. Int J Alzheimers Dis 2012:728983. doi:10.1155/2012/728983
Arduini F, Amine A, Moscone D, Palleschi G (2010) Biosensors based on cholinesterase inhibition for insecticides, nerve agents and aflatoxin B1 detection (review). Microchim Acta 170:193–214. doi:10.1007/s00604-010-0317-1
Ivanov AN, Younusov RR, Evtugyn GA, Arduini F, Moscone D, Palleschi G (2011) Acetylcholinesterase biosensor based on single-walled carbon nanotubes—Co phtalocyanine for organophosphorus pesticides detection. Talanta 85:216–221. doi:10.1016/j.talanta.2011.03.045
Nikolelis DP, Simantiraki MG, Siontorou CG, Toth K (2005) Flow injection analysis of carbofuran in foods using air stable lipid film based acetylcholinesterase biosensor. Anal Chim Acta 537:169–177. doi:10.1016/j.aca.2004.12.086
Loewenstein Y, Denari M, Zakut H, Soreq H (1993) Molecular dissection of cholinesterase domains responsible for carbamate toxicity. Chem Biol Interact 87:209–216. doi:10.1016/0009-2797(93)90044-Y
Matsumara T (1976) Toxicology of Insecticides, 2nd edn. Plenum Press, New York
Telang FW, Ding YS, Volkow ND, Molina PE, Gatley SJ (1999) Pyridostigmine, a carbamate acetylcholinesterase AChE inhibitor and reactivator, is used prophylactically against chemical warfare agents. Nucl Med Biol 26:249–250. doi:10.1016/S0969-8051(98)00094-8
Bucura B, Fournier D, Danet A, Marty JL (2006) Biosensors based on highly sensitive acetylcholinesterases for enhanced carbamate insecticides detection. Anal Chim Acta 562:115–121. doi:10.1016/j.aca.2005.12.060
Patterson TA, Kosh JW (1992) Simultaneous quantitation of arecoline, acetylcholine, and choline in tissue using gas chromatography/electron impact mass spectrometry. Biol Mass Spectrom 21:299–304. doi:10.1002/bms.1200210606
Dunphy R, Burinsky DJ (2003) Detection of choline and acetylcholine in a pharmaceutical preparation using high-performance liquid chromatography/electrospray ionization mass spectrometry. J Pharm Biomed Anal 31:905–915. doi:10.1016/S0731-7085(02)00674-X
Jin T (2003) A new fluorometric method for the detection of the neurotransmitter acetylcholine in water using a dansylcholine complex with p-sulfonated calix[8]arene. J Incl Phenom Macro 45:195–201. doi:10.1023/A:1024506714332
Bai Y, Abbott NL (2011) Recent advances in colloidal and interfacial phenomena involving liquid crystals. Langmuir 27:5719–5738. doi:10.1021/la103301d
Hussain A, Pina AS, Roque ACA (2009) Bio-recognition and detection using liquid crystals. Biosens Bioelectron 25:1–8. doi:10.1016/j.bios.2009.04.038
Han GR, Jang CH (2013) A simple strategy for detecting synthetic polymers on solid surfaces using liquid crystal. Colloid Polym Sci 291:2689–2696. doi:10.1007/s00396-013-3015-9
Hu QZ, Jang CH (2013) Spontaneous formation of micrometer-scale liquid crystal droplet patterns on solid surfaces and their sensing applications. Soft Matter 9:5779–5784. doi:10.1039/C3SM00002H
Liu D, Hu QZ, Jang CH (2013) Orientational behaviors of liquid crystals coupled to chitosan-disrupted phospholipid membranes at the aqueous-liquid crystal interface. Colloids Surf B: Biointerfaces 108:142–146. doi:10.1016/j.colsurfb.2013.02.047
Liao S, Qiao Y, Han W, Xie Z, Wu Z, Shen G, Yu R (2012) Acetylcholinesterase liquid crystal biosensor based on modulated growth of gold nanoparticles for amplified detection of acetylcholine and inhibitor. Anal Chem 84:45–49. doi:10.1021/ac202895j
Park MK, Jang CH (2010) Liquid crystal-based imaging of biomolecular interactions at roller printed protein surfaces. Bull Korean Chem Soc 31:1223–1227. doi:10.5012/bkcs.2010.31.5.1223
Park SJ, Jang CH (2014) Detection of mRNA from Escherichia coli in drinking water on nanostructured polymeric surfaces using liquid crystals. Colloid Polym Sci 292:1163–1169. doi:10.1007/s00396-014-3162-7
Han GR, Jang CH (2012) Measuring ligand-receptor binding events on polymeric surfaces with periodic wave patterns using liquid crystals. Colloids Surf B: Biointerfaces 94:89–94. doi:10.1016/j.colsurfb.2012.01.023
Han GR, Jang CH (2014) Label-free detection of viruses on a polymeric surface using liquid crystals. Colloids Surf B: Biointerfaces 116:147–152. doi:10.1016/j.colsurfb.2013.12.037
Ahn SM, Jang CH (2010) Effective parameters for the precise control of thin film buckling on elastomeric substrates. Bull Korean Chem Soc 31:419–422. doi:10.5012/bkcs.2010.31.02.419
Jang CH, Stevens BD, Carlier PR, Calter MA, Ducker WA (2002) Immobilized enzymes as catalytically-active tools for nanofabrication. J Am Chem Soc 124:12114–12115. doi:10.1021/ja017686v
Llopis X, Pumera M, Alegreta S, Merkoçi A (2009) Lab-on-a-chip for ultrasensitive detection of carbofuran by enzymatic inhibition with replacement of enzyme using magnetic beads. Lab Chip 9:213–218. doi:10.1039/b816643a
Wong L, Fisher FM (1975) Determination of carbofuran and its toxic metabolites in animal tissue by gas chromatography of their N-trifluoroacetyl derivatives. J Agric Food Chem 23:315–318. doi:10.1021/jf60198a018
Acknowledgments
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C0891) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013R1A1A1A05008333).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 712 kb)
Rights and permissions
About this article
Cite this article
Han, GR., Jang, CH. Liquid crystal sensor for the detection of acetylcholine using acetylcholinesterase immobilized on a nanostructured polymeric surface. Colloid Polym Sci 293, 2771–2779 (2015). https://doi.org/10.1007/s00396-015-3648-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3648-y