Abstract
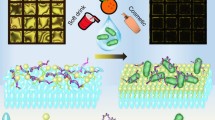
In this study, we demonstrate the detection of mRNA from Escherichia coli in drinking water using thermotropic liquid crystals (LCs). After hybridization of complementary mRNA with the single-stranded DNA immobilized on a polymer substrate containing periodic sinusoidal wave patterns, the orientation of LCs transits from a uniform to a non-uniform state, thereby inducing a change in the optical response of LCs. The periodic sinusoidal features of the polymer substrate are obtained through buckling the poly-(dimethylsiloxane) slide on a cylindrical surface, followed by replicating the associated relief structures on a poly-(urethaneacrylate) surface, where a film of gold was deposited. Then, thiol-modified single-stranded DNA was functionalized on the gold film as an mRNA receptor. The formation of mRNA–single-stranded DNA complex, which covers the sinusoidal nanostructures on the surface, induces the orientational transition of LCs. This result indicates that LCs can be used to report the specific hybridization of mRNA with single-stranded DNA, which holds promise for the sensitive and label-free detection of viable bacterial pathogens in drinking water.

ᅟ





Similar content being viewed by others
References
Gauthier F, Archibald F (2001) The ecology of “fecal indicator” bacteria commonly found in pulp and paper mill water systems. Water Res 35:2207–2218. doi:10.1016/s0043-1354(00)00506-6
Edberg SC, Allen MJ, Smith DB (1988) National field evaluation of a defined substrate method for the simultaneous enumeration of total coliforms and Escherichia coli from drinking water: comparison with the standard multiple tube fermentation method. Appl Environ Microbiol 54:1595–1601
Lazcka O, Del Campo FJ, Muñoz FX (2007) Pathogen detection: a perspective of traditional methods and biosensors. Biosens Bioelectron 22:1205–1217. doi:10.1016/j.bios.2006.06.036
Koets M, Van der Wijk T, Van Eemeren JTWM, Van Amerongen A, Prins MWJ (2008) Rapid DNA multi-analyte immunoassay on a magneto-resistance biosensor. Biosens Bioelectron 24:1893–1898. doi:10.1016/j.bios.2008.09.023
Sadik OA, Aluoch AO, Zhou A (2009) Status of biomolecular recognition using electrochemical techniques. Biosens Bioelectron 24:2749–2765. doi:10.1016/j.bios.2008.10.003
Keer JT, Birch L (2003) Molecular methods for the assessment of bacterial viability. J Microbiol Methods 53:175–183. doi:10.1016/s0167-7012(03)00025-3
Elsholz B, Worl R, Blohm L, Albers J, Feucht H, Grunwald T, Jurgen B, Schweder T, Hintsche R (2006) Automated detection and quantitation of bacterial RNA by using electrical microarrays. Anal Chem 78:4794–4802. doi:10.1021/ac0600914
Fang Z, Kelley SO (2009) Direct electrocatalytic mRNA detection using PNA-nanowire sensors. Anal Chem 81:612–617. doi:10.1021/ac801890f
Sheridan GEC, Masters CI, Shallcross JA, Mackey BM (1998) Detection of mRNA by reverse transcription-PCR as an indicator of viability in Escherichia coli cells. Appl Environ Microbiol 64:1313–1318
Simpkins SA, Chan AB, Hays J, Pöpping B, Cook N (2000) An RNA transcription-based amplification technique (NASBA) for the detection of viable Salmonella enterica. Lett Appl Microbiol 30:75–79. doi:10.1046/j.1472-765x.2000.00670.x
Sanvicens N, Pastells C, Pascual N, Marco M-P (2009) Nanoparticle-based biosensors for detection of pathogenic bacteria. Trends Anal Chem 28:1243–1252. doi:10.1016/j.trac.2009.08.002
Conde J, de la Fuente JM, Baptista PV (2010) RNA quantification using gold nanoprobes—application to cancer diagnostics. J Nanobiotechnol 8:5. doi:10.1186/1477-3155-8-5
Gupta VK, Skaife JJ, Dubrovsky TB, Abbott NL (1998) Optical amplification of ligand-receptor binding using liquid crystals. Science 279:2077–2080. doi:10.1126/science.279.5359.2077
Brake JM, Mezera AD, Abbott NL (2003) Effect of surfactant structure on the orientation of liquid crystals at aqueous–liquid crystal interfaces. Langmuir 19:6436–6442. doi:10.1021/la034132s
Hu QZ, Jang CH (2012) Using liquid crystals to report molecular interactions between cationic antimicrobial peptides and lipid membranes. Analyst 137:567–570. doi:10.1039/c1an15743d
Hu QZ, Jang CH (2012) Using liquid crystals for the label-free detection of catalase at aqueous–LC interfaces. J Biotechnol 157:223–227. doi:10.1016/j.jbiotec.2011.11.010
Gu Y, Nederberg F, Kange R, Shah RR, Hawker CJ, Moller M, Hedrick JL, Abbott NL (2002) Anchoring of liquid crystals on surface-initiated polymeric brushes. Chem Phys Chem 5:448–451. doi:10.1002/1439-7641(20020517)3:5<448::AID-CPHC448>3.0.CO;2-0
Luk YY, Tingey ML, Hall DJ, Israel BA, Murphy CJ, Bertics PJ, Abbott NL (2003) Using liquid crystals to amplify protein–receptor interactions: design of surfaces with nanometer-scale topography that present histidine-tagged protein receptors. Langmuir 19:1671–1680. doi:10.1021/la026152k
Kim SR, Shah RR, Abbott NL (2000) Orientations of liquid crystals on mechanically rubbed films of bovine serum albumin: a possible substrate for biomolecular assays based on liquid crystals. Anal Chem 72:4646–4653. doi:10.1021/ac000256n
Jang CH, Tingey ML, Korpi NL, Wiepz GJ, Schiller JH, Bertics PJ, Abbott NL (2005) Using liquid crystals to report membrane proteins captured by affinity microcontact printing from cell lysates and membrane extracts. J Am Chem Soc 127:8912–8913. doi:10.1021/ja051079g
Bi X, Lai SL, Yang KL (2009) Liquid crystal multiplexed protease assays reporting enzymatic activities as optical bar charts. Anal Chem 81:5503–5509. doi:10.1021/ac900793w
Tan H, Yang S, Shen G, Yu R, Wu Z (2010) Signal-enhanced liquid-crystal DNA biosensors based on enzymatic metal deposition. Angew Chem Int Ed 49:8608–8611. doi:10.1002/anie.201004272
Park SJ, Jang CH (2010) Using liquid crystals to detect DNA hybridization on polymeric surfaces with continuous wavy features. Nanotechnology 21:425502–425508. doi:10.1088/0957-4484/21/42/425502
Lai SL, Tan WL, Yang KL (2011) Detection of DNA targets hybridized to solid surfaces using optical images of liquid crystals. ACS Appl Mater Interfaces 3:3389–3395. doi:10.1021/am200571h
Han GR, Jang CH (2012) Measuring ligand–receptor binding events on polymeric surfaces with periodic wave patterns using liquid crystals. Colloids Surf B: Biointerfaces 94:89–94. doi:10.1016/j.colsurfb.2012.01.023
Ahn SM, Jang CH (2010) Effective parameters for the precise control of thin film buckling on elastomeric substrates. Bull Korean Chem Soc 31:419–422. doi:10.5012/bkcs.2010.31.02.419
Hu QZ, Jang CH (2011) Liquid crystal-based sensors for the detection of heavy metals using surface-immobilized urease. Colloids Surf B: Biointerfaces 88:622–626. doi:10.1016/j.colsurfb.2011.07.052
Acknowledgments
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2013R1A1A1A05008333) and a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C0891).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, SJ., Min, J., Hu, QZ. et al. Detection of mRNA from Escherichia coli in drinking water on nanostructured polymeric surfaces using liquid crystals. Colloid Polym Sci 292, 1163–1169 (2014). https://doi.org/10.1007/s00396-014-3162-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-014-3162-7