Abstract
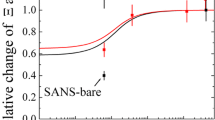
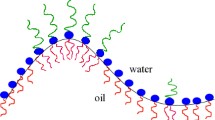
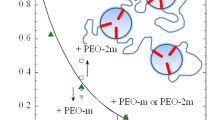
After linear hydrophilic polymers with a short hydrophobic sticker have been proven to be highly suitable as efficiency boosters in microemulsions, a whole set of asymmetric polymers was studied in more detail. Sticker polymers with two hydrophilic polymeric arms, hydrophobic polymers with ionic stickers, and a Y-shaped tri-arm-polymer were studied for their high surface activity. While low asymmetry of the Y-shaped polymer resulted in very similar dependence as a diblock copolymer, the highly asymmetric polymers showed a renormalized behavior of the bending rigidity coming from preferential accretion of the polymer at the membrane. The experimental evidence for the accretion of the polymer according to the preferential membrane bending is reported here for the first time. Furthermore, the isolated, diffuse counter ion clouds of the ionic sticker polymers act in a way similar to that of a polymer anchored at the membrane. All asymmetric polymers are highly suitable as efficiency boosters in microemulsions.














Similar content being viewed by others
References
Jakobs B, Sottmann T, Strey R, Allgaier J, Willner L, Richter D (1999) Amphiphilic block copolymers as efficiency boosters for microemulsions. Langmuir 15:6707–6711
Endo H, Mihailescu M, Monkenbusch M, Allgaier J, Gompper G, Richter D, Jakobs B, Sottmann T, Strey R, Grillo I (2001) Effect of amphiphilic block copolymers on the structure and phase behavior of oil–water-surfactant mixtures. J Chem Phys 115:580–600
Maccarrone S, Byelov D, Auth T, Allgaier J, Frielinghaus H, Gompper G, Richter D (2013) Confinement effects in block copolymer modified bicontinuous microemulsions. J Phys Chem B 117:5623–5632
Frank C, Frielinghaus H, Allgaier J, Richter D (2008) Hydrophilic alcohol ethoxylates as efficiency boosters for microemulsions. Langmuir 24:6036–6043
Helfrich WZ (1973) Elastic properties of lipid bilayers—theory and possible experiments. Naturforschung 28c:693–703
Peliti L, Leibler S (1985) Effects of thermal fluctuations on systems with small surface-tension. Phys Rev Lett 54:1690–1693
Hiergeist C, Lipowsky R (1996) Elastic properties of polymer-decorated membranes. J Phys II France 6:1465–1481
Eisenriegler E, Hanke A, Dietrich S (1996) Polymers interacting with spherical and rodlike particles. Phys Rev E 54:1134–1152
Byelov D (2003) A SANS study of microemulsions with polymer additions. WestfälischeWilhelms-Universität, Münster
Kawaguchi S, Imai G, Suzuki J, Miyahara A, Kitano T, Ito K (1997) Aqueous solution properties of oligo-and poly (ethylene oxide) by static light scattering and intrinsic viscosity. Polymer 38:2885–2891
Fetters LJ, Lohse DJ, Colby RH (2007) Chain dimensions and entanglement spacings. In: Mark JE (ed) Physical properties of polymers handbook, 2nd edn. Springer, New York, pp 447–454
Gompper G, Kroll DM (1998) Membranes with fluctuating topology: Monte Carlo simulations. Phys Rev Lett 81:2284–2287
Strey R (1994) Microemulsion microstructure and interfacial curvature. Colloid Polym Sci 272:1005–1019
Sottmann T, Strey R (1997) Ultralow interfacial tensions in water–n-alkane–surfactant systems. J Chem Phys 106:8606–8615
Pedersen JS, Posselt D, Mortensen K (1990) Analytical treatment of the resolution function for small-angle scattering. J Appl Cryst 23:321–333
Teubner M, Strey R (1987) Origin of the scattering peak in microemulsions. J Chem Phys 87:3195–3200
Pieruschka P, Safran SA (1993) Random interfaces and the physics of microemulsions. Europhys Lett 22:625–630
Pieruschka P, Safran SA (1995) Random interface model of sponge phases. Europhys Lett 31:207–212
Peltomäki M, Gompper G, Kroll D (2012) Scattering intensity of bicontinuous microemulsions and sponge phases. J Chem Phys 136:134708
Holderer O, Frielinghaus H, Monkenbusch M, Klostermann M, Sottmann T, Richter D (2013) Experimental determination of bending rigidity and saddle splay modulus in bicontinuous microemulsions. Soft Matter 9:2308–2313
Zilman AG, Granek R (1996) Undulations and dynamic structure factor of membranes. Phys Rev Lett 77:4788–4791
Mihailescu M, Monkenbusch M, Endo H, Allgaier J, Gompper G, Stellbrink J, Richter D, Jakobs B, Sottmann T, Farago B (2001) Dynamics of bicontinuous microemulsion phases with and without amphiphilic block-copolymers. J Chem Phys 115:9563–9577
Kahlweit M, Strey R (1985) Phase behavior of ternary systems of the type H2O-oil-nonionic amphiphile (microemulsions). Angew Chem Int Ed Engl 24:654–668
Kahlweit M, Strey R, Firman P (1986) Search for tricritical points in ternary systems: water–oil-nonionic amphiphile. J Phys Chem 90:671–677
Lekkerkerker HNW, Kegel WK, Overbeek JTG (1996) Phase behavior of ionic microemulsions. Bunsenges Ber Phys Chem 100:206–217
Lekkerkerker HNW (1989) Contribution of the electric double layer to the curvature elasticity of charged amphiphilic monolayers. Phys A 159:319–328
Lipowsky R, Döbereiner H-G, Hiergeist C, Indrani V (1998) Membrane curvature induced by polymers and colloids V. Phys A 249:536–543
Gov N, Safran SA (2004) Pinning of fluid membranes by periodic harmonic potentials. Phys Rev E 69:011101
Bickel T, Jeppesen C, Marques CM (2001) Local entropic effects of polymers grafted to soft interfaces. Eur Phys J E 4:33–43
Breidenich M, Netz N, Lipowsky R (2000) The shape of polymer-decorated membranes. Europhys Lett 49:431–437
Auth T, Gompper G (2003) Self-avoiding linear and star polymers anchored to membranes. Phys Rev E 68:051801
Auth T, Gompper G (2005) Fluctuation spectrum of membranes with anchored linear and star polymers. Phys Rev E 72:031904
Schwindeman, J. A. U.S. Patent 5,321,148 (1994)
Acknowledgement
This work utilized facilities supported in part by the National Science Foundation under Agreement No. DMR-0944772.
Conflict of interest
The authors declare no conflict of interest. The identification of any commercial product or trade name does not imply endorsement or recommendation by the National Institute of Standards and Technology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Online Resource. Five figures to support the information about the synthesis of the Y-shaped polymer.
Figure S1a
(PDF 43 kb)
Figure S1b
(PDF 38 kb)
Figure S2
(PDF 47 kb)
Figure S3
(PDF 111 kb)
Figure S4
(PDF 81 kb)
Figure S5
(PDF 27 kb)
Appendix A
Appendix A
The PEP-(PEO)2 star-block copolymer was prepared by a five-step process. A detailed reaction scheme is presented in Online Resource 1. The first step of the synthesis includes the anionic polymerization of isoprene using t-butyllithium as initiator and benzene as polymerization solvent. The living polyisoprene (PI) chains were endcapped with trichloromethylsilane. Thereby, a large excess of the chlorosilane linking agent was used such that multiple substitution could be essentially avoided. Thus, the resulting PI polymer still carried two remaining chloro functions in terminal position, PI-Si(Me)-Cl2. In a subsequent second step the difunctional PI-Si(Me)-Cl2 was reacted with a small excess of the organolithium compound 3-(t-butyldimethylsilyloxy)-1-propyllithium. This material, usually used as an OH-protected functionalized initiator for anionic polymerization, was prepared according to a procedure described by Schwindeman [33]. The reaction with the difunctional PI-Si(Me)-Cl2 yielded the corresponding PI-Si(Me)-(3-(t-butyldimethylsilyloxy-1-propyl))2 which was isolated by precipitation in methanol. In the third step, the OH-functionalized PI was converted to poly(ethylene-alt-propylene) (PEP) by catalytic hydrogenation using an unreduced Pd on Bariumsulfate catalyst in heptane at 80 °C. In a fourth step, the difunctionalized PEP polymer was deprotected from the t-butyldimethylsilyloxy-groups. This was achieved by adding a slight stoichiometric excess of 1.25 m hydrochloric acid to a polymer solution in THF and stirring at room temperature for more than 4 h. The deprotected PI-Si(Me)-(3-hydroxy-1-propyl)2 was again isolated by precipitating in methanol. The polymer was dried in high vacuum for several days in order remove all volatile protic impurities. Finally, in the fifth step, the hydroxy groups of the functionalized PEP polymer were transformed to the corresponding potassium analog PI-Si(Me)-(3-(KO-1-propyl))2. This was achieved by titration with freshly prepared potassium naphthalenide solution in THF. The potassium compound was then taken as macroinitiator for the polymerization of ethylene oxide. The polymerization was performed at 50 °C for 24 h and then terminated with acetic acid yielding terminal OH groups. The resulting PEP-(PEO)2 star-block copolymer was precipitated in acetone at −20 °C and finally freeze-dried from benzene in high vacuum. The final PEP(PEO)2 star-block copolymer as well as the precursors were characterized by size exclusion chromatography (SEC) and 1H-NMR. SEC-chromatograms were obtained with a PL GPC 220 from Polymer Labs equipped with a refractive index detector. Three Polypore (Polymer Labs) columns with a continuous pore size distribution were used with a 85/15 mixture of tetrahydrofuran and N,N-dimethylacetamide as eluent at 50 °C. Molecular weight distributions were calculated either from PEO-calibration (PEP(PEO)2) or PS-calibration (PI and PEP). 1H-NMR spectra were recorded with a Varian Inova 400-MHz spectrometer. All samples were measured at 295 K in CDCl3 with 5-mm PFG Auto X DB Probe. A small sample of the uncapped PI polymer was removed as reference material for separate characterization. The number average molecular weight, Mn = 5,240 g/mol, was calculated from the NMR spectrum taking the nine protons from the t-butyl initiator group as internal standard. The molecular weight distribution, M w/M n, was smaller than 1.03 as calculated from SEC data using PS calibration. SEC-chromatograms of functionalized and unfunctionalized PI are shown in Online Resource 2, corresponding 1H-NMR spectra in Online Resource 3. From the NMR spectra, by comparing the integrals of the t-butyl group of the initiator with those of the protecting group, one can conclude that the functionalization is close to 100 %. SEC data, however, reveal a small fraction of a polymer with higher molecular weight, most likely due to a double substitution of living PI chains to the crosslinking agent. The molecular weight is increased by the protecting group as can be seen from the slight shift of the SEC curve. The corresponding number average molecular weight is calculated to be Mn = 5630 g/mol. The completeness of the hydrogenation was verified by the disappearance of vinyl-protons in the 1H-NMR-spectrum, which further indicates that the functional groups remained unaffected by the saturation process (Online Resource 4). The deprotection from the t-butyldimethylsilyl-group was monitored by 1H-NMR by following the disappearance of the 12 protons for the four methyl-groups at 0.03 ppm (see Fig. S3). Complete deprotection was achieved after 4 h. SEC-data of the PEP precursor after deprotection ((3-hydroxy-1-propyl)2-Si(Me)-PEP) and of the final product (PEP(PEO)2) are shown in Online Resource 5. The SEC data of PEP-(PEO)2 revealed a narrow molecular weight distribution of M w/M n<1.06. Small fractions of high and low molecular weight byproducts (≈5 %) additionally appear in the chromatograms. For the purpose of this work, however, these small amounts of impurities were neglected and the origin not further explored. The 1H-NMR -spectrum of the final product is shown in Online Resource 6. Molecular weights of the PEO chains were calculated from the integrals of the PEO-methylene protons at ≈3.5 to 3.9 ppm relative to the aliphatic signals between 0.7 and 1.5 ppm of the PEP polymer with known molecular weight. Thus, the PEO number average molecular weight was determined to be Mn = 14250 g/mol (7125 g/mol/PEO-chain) which gives an overall M n of ≈19.8 kg/mol for the PEP(PEO)2 star-block copolymer.
Rights and permissions
About this article
Cite this article
Brodeck, M., Maccarrone, S., Saha, D. et al. Asymmetric polymers in bicontinuous microemulsions and their accretion to the bending of the membrane. Colloid Polym Sci 293, 1253–1265 (2015). https://doi.org/10.1007/s00396-014-3449-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-014-3449-8