Abstract
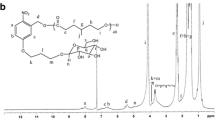
Porphyrin-cored poly(l-lactide) (SPPLA) was successfully synthesized from ring-opening polymerization (ROP) of l-lactide initiated with porphyrin core. Then, SPPLA was coupled with benzylsulfanylthiocarbonylsufanylpropionic acid (BSPA), and a macro-reversible addition-fragmentation chain transfer (macroRAFT) polymerization agent SPPLA-BSPA was obtained. Finally, star-shaped porphyrin-cored poly(l-lactide)-b-poly(gluconamidoethyl methacrylate) (SPPLA-b-PGAMA) block copolymers were synthesized via RAFT of unprotected gluconamidoethyl methacrylate (GAMA) in 1-methyl-2-pyrrolidinone (NMP) solution at 70 °C. The structure of this block copolymer was thoroughly studied by nuclear magnetic resonance spectroscopy (NMR), gel permeation chromatography (GPC), and differential scanning calorimetry (DSC). Under the irradiation, such SPPLA-b-PGAMA copolymer exhibits efficient singlet oxygen generation and indicates high fluorescence quantum yields. Notably, with UV–vis and dynamic light scattering (DLS) analysis, SPPLA-b-PGAMA showed a very specific recognition with concanavalin A (ConA). Particularly, MTT shows that the cytotoxicity of SPPLA-b-PGAMA against COS-7 cells was very low and, when given a longer irradiation time, more BEL-7402 cancer cells died, which will be investigated in this study.













Similar content being viewed by others
References
Huang Z (2005) Technol Cancer Res Treat 4:283
Anand S, Ortel BJ, Pereira SP, Hasan T, Maytin EV (2012) Cancer Lett 326:8–16
Dolmans DE, Fukumura D, Jain RK (2003) Nat Rev Cancer 3:380
Snyder JW, Greco WR, Bellnier DA, Vaughan L, Henderson BW (2003) Cancer Res 63:8126
Zhang XM, Wu HS, Chen XM (2003) Eur J Inorg Chem 2003:2959
Nishiyama N, Stapert HR, Zhang GD et al (2003) Bioconjug Chem 14:58
Dai X-H, Zhang H-D, Dong C-M (2009) Polymer 50:4626
Peng C-L, Shieh M-J, Tsai M-H, Chang C-C, Lai P-S (2008) Biomaterials 29:3599
Dai XH, Dong CM (2008) J Polym Sci A Polym Chem 46:817
Murariu M, Ferreira AD, Alexandre M, Dubois P (2008) Polym Adv Technol 19:636. doi:10.1002/pat.1131
Hu Y, Hu Y, Topolkaraev V, Hiltner A, Baer E (2003) Polymer 44:5711
Jiang L, Wolcott MP, Zhang J (2006) Biomacromolecules 7:199
Cohn D, Hotovely-Salomon A (2005) Polymer 46:2068
Maglio G, Migliozzi A, Palumbo R (2003) Polymer 44:369
Riley T, Stolnik S, Heald C et al (2001) Langmuir 17:3168
Hecht S, Vladimirov N, Frechet JM (2001) J Am Chem Soc 123:18
Hsu C-Y, Nieh M-P, Lai P-S (2012) Chem Commun 48:9343
Dai XH, Liu W, Huang YF, Dong CM (2011) Adv Mater Res 239:1703
Dai X-H, Dong C-M, Fa H-B, Yan D, Wei Y (2006) Biomacromolecules 7:3527
Spain SG, Gibson MI, Cameron NR (2007) J Polym Sci A Polym Chem 45:2059
Kiessling LL, Gestwicki JE, Strong LE (2006) Angew Chem Int Ed 45:2348
Bertozzi CR, Kiessling LL (2001) Science 291:2357
Ladmiral V, Melia E, Haddleton DM (2004) Eur Polym J 40:431
Miura Y (2012) Polym J 44:679. doi:10.1038/pj.2012.4
Thoma G, Patton JT, Magnani JL, Ernst B, Öhrlein R, Duthaler RO (1999) J Am Chem Soc 121:5919
Okada M (2002) Prog Polym Sci 27:87
Wang Q, Dordick JS, Linhardt RJ (2002) Chem Mater 14:3232
Ballut S, Makky A, Loock B, Michel JP, Maillard P, Rosilio V (2009) Chem Commun 2:224
Laville I, Pigaglio S, Blais JC et al (2006) J Med Chem 49:2558
Maillard P, Loock B, Grierson D et al (2007) Photodiagn Photodyn Ther 4:261
Zhu G, Mallery SR, Schwendeman SP (2000) Nat Biotechnol 18:52
Li K, Liu B (2010) Polym Chem 1:252
Nederberg F, Connor EF, Möller M, Glauser T, Hedrick JL (2001) Angew Chem Int Ed 40:2712
Myers M, Connor EF, Glauser T, Möck A, Nyce G, Hedrick JL (2002) J Polym Sci A Polym Chem 40:844
Discher DE, Ahmed F (2006) Annu Rev Biomed Eng 8:323
Ren T, Wang A, Yuan W, Li L, Feng Y (2011) J Polym Sci A Polym Chem 49:2303
Li ZY, Wang HY, Li C et al (2011) J Polym Sci A Polym Chem 49:286
Quimby DJ, Longo FR (1975) J Am Chem Soc 97:5111
Ye S, Czuba M, Romiszewska A, Karolczak J, Graczyk A (2002) Opt Appl 33:489
Spiller W, Kliesch H, Woehrle D, Hackbarth S, Roeder B, Schnurpfeil G (1998) J Porphyrins Phthalocyanines 2:145
Tada DB, Vono LL, Duarte EL et al (2007) Langmuir 23:8194
Gerhardt SA, Lewis JW, Zhang JZ, Bonnett R, McManus KA (2003) Photochem Photobiol Sci 2:934
Park SY, Han BR, Na KM, Han DK, Kim SC (2003) Macromolecules 36:411cbrs5
Madbouly SA, Xia Y, Kessler MR (2012) Macromolecules 45:7729. doi:10.1021/ma301458n
Grubbs RB (2011) Polym Rev 51:104. doi:10.1080/15583724.2011.566405
Siegwart DJ, Oh JK, Matyjaszewski K (2012) Prog Polym Sci 37:18. doi:10.1016/j.progpolymsci.2011.08.001
Gregory A, Stenzel MH (2012) Prog Polym Sci 37:38. doi:10.1016/j.progpolymsci.2011.08.004
Albertin L, Stenzel MH, Barner-Kowollik C, Foster LJR, Davis TP (2005) Macromolecules 38:9075
Bernard J, Favier A, Zhang L et al (2005) Macromolecules 38:5475
Dong CM, Guo YZ, Qiu KY, Gu ZW, Feng XD (2005) J Control Release 107:53
Ideta R, Tasaka F, Jang WD et al (2005) Nano Lett 5:2426
Feng H, Dong CM (2006) Biomacromolecules 7:3069. doi:10.1021/bm060568l
Haag R (2004) Angew Chem-Int Edit 43:278. doi:10.1002/anie.200301694
Moan J (1984) Photochem Photobiol 39:445
Choi K-H, Wang K-K, Shin EP et al (2011) J Phys Chem C 115:3212
Ringot C, Sol V, Barrière M et al (2011) Biomacromolecules 12:1716
Acknowledgments
The authors are greatly grateful for the financial support of the National Natural Science Foundation of China (21004031), the Natural Science Foundation of Jiangsu Province (BK2011459), the National Postdoctoral Foundation of China (20090461065), the National Postdoctoral Foundation of Jiangsu Province (1001034B), Open Foundation of Stake Key Laboratory of Natural and Biomimetic Drugs, Peking University (K20110105), and the social development Foundation of Zhen jiang (SH2012024).
Author information
Authors and Affiliations
Corresponding author
Additional information
Mr Pan has done a prominent work in the study of singlet oxygen research and Fluorescence quantum yield.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 93 kb)
Rights and permissions
About this article
Cite this article
Dai, XH., Wang, ZM., Liu, W. et al. Biomimetic star-shaped porphyrin-cored poly(l-lactide)-b-glycopolymer block copolymers for targeted photodynamic therapy. Colloid Polym Sci 292, 2111–2122 (2014). https://doi.org/10.1007/s00396-014-3244-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-014-3244-6