Abstract
A series of CoIII carboxylate based upon N,N,O,O-tetradentate Schiff base ligand framework have been prepared. X-ray diffraction analysis confirms that these Schiff base CoIII carboxylate are all monomeric species with a six-coordinated central Co in their solid structures. The activities and polycarbonate selectivity of these complexes toward the copolymerization of epoxide (cyclohexene oxide and propylene oxide) and carbon dioxide have been investigated in the presence of bis(triphenylphosphine)iminium chloride. Copolymerization experiments indicate that [bis(α-methyl-3,5-di-tertbutyl-salicylaldehyde) ethylenediiminato] CoIIIOOCH3 exhibits the highest activity and polycarbonate selectivity among these CoIII carboxylate. The resultant copolymer contained almost 100 % carbonate linkages with the molecular weight up to 71.8 kg mol−1 as well as narrow polymer dispersity index (polymer dispersity index = 1.5). The substituents and the mode of the bridging part between the two nitrogen atoms both exert significant influences upon the progress of the copolymerizations, influencing both the polycarbonate selectivity and the rate of copolymerization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The transition metal complexes with Schiff bases as ligands are promising systems for application in the coupling of CO2 with epoxide to aliphatic polycarbonates because the copolymerization of CO2 and epoxide [such as cyclohexene oxide (CHO), propylene oxide (PO)] with discrete metal complex has been well documented [1]. Copolymers of CHO with CO2 are important product with favorable property in comparison to homopoly(cyclohexene oxide), which is also one of the most promising routes for the chemical fixation of carbon dioxide [2–4]. For another, introduction of a CO2 comonomer to the poly(cyclohexene oxide) chain changes the structure, and consequently, the properties of the polymer product are obtained. The effect is dependent on the type of catalytic system used and polymerization conditions [5].
Metal salen complexes have been used for the copolymerization of CO2 with certain monomers to form polycarbonates [6–9]. A particular feature of these complexes is that polymers tend to be isolated with very narrow molecular weight distributions and that the molecular weights of the produced copolymer can be controlled. Typically, these complexes are tolerant toward water and air, so that extensive drying and purification of reagents are unnecessary. (Salen)CrIIIX or (salen)CoIIIX complexes (salen=N,N′-bis(salicylidene)-1,2-diaminoalkane), which exhibit outstanding performance and can obtain the carbonate contents reach almost 100 %, are composed of zinc acetate complexes [10], tetrakis(pentafluorophenyl)porphyrin chromium(III) chloride [11] and β-diiminate zinc complexes [12, 13]. (Salen)CrIIIX [14–18] or (Salen)CoIIIX [19–25] complexes [salen = N,N′-bis(salicylidene)-1,2-diaminoalkane] exhibit outstanding performance, and the carbonate contents reach almost 100 %. These studies should be very important for designing a highly efficient complex and for understanding the scope and the limitation of these types of transition metal complexes as CHO/CO2 copolymerization catalysts.
Metal salen complexes have received increasing attention for their unusually high catalytic activity on the copolymerization of CO2 with epoxide [1]. The influence of the phenolate ortho and para substituents as well as the axial group epoxide/CO2 copolymerization has been studied widely [6, 26, 27]. The cobalt Schiff base complex with axial acetate ligand has been employed to the carbon dioxide fixation field. However, some thought one cobaltate complex with one axial acetate ligand and others reported cobaltate complex with two axial acetate ligands [22, 23, 28–33]. Coates and coworkers [34] reported that a five-coordinated cobalt complex is pseudo square-pyramidal with Co located slightly above the plane of the salen ligand in the direction of the axial ligand. Recently, we reported the X-ray crystal structure of the salen CoIII(2,4-dinitrophenoxy) is six coordinated [35, 36]. As far as we are aware, the crystal structure of the cobalt Schiff base complexes with axial acetate ligand has not been reported.
In the present work, a series of novel α-methyl ligated cobaltIII carboxylate Schiff bases complexes were developed to synthesis the alternating copolymer of CHO/CO2 with an ionic quaternary ammonium salt bis(triphenylphosphine)iminium chloride [PPN]Cl as a cocatalyst. The influences of the ligand framework and the reaction conditions on the catalytic activities of the related complexes will be explicated. Based on the X-ray crystal structure and results of copolymerization of CO2 with CHO, the copolymerization mechanism is examined as well.
Experimental section
General methods
All manipulations involving air- and/or water-sensitive compounds were carried out using standard Schlenk techniques under dry argon. CHO was refluxed over CaH2, and fractionally distilled under an argon atmosphere prior to use. CO2 (99.995 %) was purchased from Qingdao Institute of Special Gases and used as received. Diethyl ether was distilled from sodium benzophenone. Methylene chloride, hexane, and chloroform were distilled from CaH2 under argon. All ligands were synthesized according to modified literature procedures [37–39].
Measurements
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AV 300M instrument at room temperature. Chemical shifts were given in parts per million from tetramethylsilane. Gel permeation chromatography (GPC) measurements were carried out with a Waters instrument (515 HPLC pump) equipped with a Wyatt interferometric refractometer. GPC columns were eluted with THF at 25 °C at 1 ml min−1. The molecular weights were calibrated against polystyrene standards. IR spectra were recorded on a Perkin-Elmer 2000 FTIR spectrometer. matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF) MS analyses were carried out on a commercial Reflex III MALDI-TOF mass spectrometer (Bruker Co., Germany) equipped with delayed extraction technology. Ions formed by a pulsed UV laser beam with 3 nm pulse (nitrogen laser, λ = 337 nm) were accelerated through 20 kV, and detection voltage was set at 1.60 kV. The laser was adjusted by the experiments slightly above the threshold, and the mass spectra were obtained from the results of 35 laser shots in positive mode. The matrix, trans-3-indoleacrylic acid, was dissolved in purified THF (0.1 mol l−1), and the solution was mixed with the polymer solution in THF (5 mg ml−1) in a 1:1 v/v ratio. Crystallographic data were collected on a Bruker APEX CCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at 293 K. The structure was refined by the full-matrix least-squares method on F 2 using the SHELXTL-97 crystallographic software package. Anisotropic thermal parameters were used to refine all nonhydrogen atoms. Hydrogen atoms were located in idealized positions.
Synthesis of complexes 1–6
Synthesis of complex 1
To a stirred mixture of salen CoII complex (1.16 g, 2.0 mmol) in CH2Cl2 (150 ml), the mixture of CH3COOH (0.12 g, 2.0 mmol, 1 equiv.) and CH2Cl2 (20 ml) was added. The solution was stirred under dry oxygen atmosphere at room temperature for 60 min. The solvents were removed in vacuum to leave a crude green dark solid in near 100 % yield. The resulted solid was further treated with the mixture of diethyl ether and hexane, and then dried at 60 °C in vacuum for 24 h. Yield: 89 %. Elemental analysis calcd (%) for C36H53CoN2O4: C, 67.90; H, 8.39; N, 4.40. Found: C, 67.87; H, 8.35; N, 4.33. 1H NMR (1,4-Dioxane-d8): 1.12(s, 6H), 1.32 (s, 18H), 1.74 (s, 18H), 2.14 (s, 2H), 4.13 (m, 4H), 7.41 (s, 2H), 7.49 (s, 2H). 13C NMR (1,4-Dioxane-d8): δ 19.02, 27.14, 23.57, 30.35, 31.47, 32.48, 40.71, 124.06, 121.22, 126.48, 128.98, 137.48, 142.41, 163.69, 176.27. ESIMS (m/z, M+ + H): 577.3201; Calcd. for [C34H50N2O2Co]+: 577.3204.
Synthesis of complex 2
It was synthesized according to the literature method [35]. Yield: 91 %. Elemental analysis calcd (%) for C34H49CoN2O4: C, 67.09; H, 8.11; N, 4.60. found: C, 67.05; H, 8.04; N, 4.55. 1H NMR (1,4-Dioxane-d8): 1.31 (s, 18H), 1.73 (s, 18H), 2.09 (s, 3H), 4.12 (m, 4H), 7.39 (s, 2H), 7.45 (s, 2H). 8.12 (s, 2H). 13C NMR (1,4-Dioxane-d8): δ 24.56, 30.75, 30.27, 31.48, 33.49, 58.25, 122.16, 125.42, 127.37, 137.72, 142.41, 151.35, 162.69, 168.70. ESIMS (m/z, M+ + H): 549.2890; Calcd. for [C32H46N2O2Co]+: 549.2891.
Synthesis of complex 3
It was synthesized as a similar procedure of complex 1. Yield: 85 %. Elemental analysis calcd (%) for C28H35CoI2N2O4: C, 43.32; H, 4.54; N, 3.61; found: C, 43.28; H, 4.51; N, 3.60. 1H NMR (1,4-Dioxane-d8): 1.10 (s, 6H), 1.37 (s, 18H), 2.09 (s, 3H), 4.02 (m, 4H), 7.49 (s, 2H), 7.55 (s, 2H). 13C NMR (1,4-Dioxane-d8): δ 20.56, 29.67, 30.15, 32.17, 125.16, 128.42, 138.37, 137.12, 151.35, 163.59, 169.71. ESIMS (m/z, M+ + H): 716.9882; Calcd. for [C26H32I2N2O2Co]+: 716.9885.
Synthesis of complex 4
It was synthesized as a similar procedure of complex 1. Yield: 88 %. Elemental analysis calcd (%) for C28H35CoI2N2O4: C, 43.32; H, 4.54; N, 3.61; found: C, 43.29; H, 4.50; N, 3.59. 1H NMR (1,4-Dioxane-d8): 1.06 (s, 6H), 1.31 (s, 18H), 2.07 (s, 3H), 4.05 (m, 4H), 7.45 (s, 2H), 7.51 (s, 2H). 13C NMR (1,4-Dioxane-d8): δ 20.16, 25.63, 30.25, 31.45,40.15, 89.16, 125.92, 126.37, 139.98, 144.34, 1631.35, 164.59, 169.78. ESIMS (m/z, M+ + H): 716.9880; Calcd. for [C26H32I2N2O2Co]+: 716.9885.
Synthesis of complex 5
It was synthesized as a similar procedure of complex 1. Yield: 87 %. Elemental analysis calcd (%) for C20H17CoI4N2O4: C, 26.23; H, 1.87; N, 3.06. found: C, 26.20; H, 1.85; N, 3.07. 1H NMR (1,4-Dioxane-d8): 1.08 (s, 6H), 2.09 (s, 3H), 4.07 (m, 4H), 7.76 (s, 2H), 7.79 (s, 2H). 13C NMR (1,4-Dioxane-d8): δ 21.34, 24.63, 29.24, 89.16, 91.21, 128.12, 136.37, 139.98, 147.74, 1661.31, 165.59, 169.89. ESIMS (m/z, M+ + H): 856.6564; Calcd. for [C18H14I4N2O2Co]+: 856.6566.
Synthesis of complex 6
It was synthesized according to the literature method [35]. Yield: 89 %. Elemental analysis calcd (%) for C37H55CoN2O4: C, 68.29; H, 8.52; N, 4.30; found: C, 67.32; H, 8.67; N, 4.51. 1H NMR (1,4-Dioxane-d8): 1.35 (s, 18H), 1.37 (s, 18H), , 1.65 (s, 6H), 2.09 (s, 3H), 7.64 (s, 2H), 7.69 (s, 2H). 13C NMR (1,4-Dioxane-d8): δ 24.54, 30.13, 31.25, 32.46, 31.66, 62.71, 128.12, 128.56, 137.37, 139.88, 145.94, 154.54, 164.37, 170.45. ESIMS (m/z, M+ + H): 591.3363; Calcd. for [C35H52N2O2Co]+: 591.3361.
Representative copolymerization procedure
A 100-ml stainless steel autoclave equipped with a magnetic stirring bar was heated to 120 °C under vacuum for 2 h. After cooled to room temperature, the autoclave was charged with CO2. A mixture of complex and [PPN]Cl was dissolved in 1,4-dioxane under CO2 atmosphere. The mixture solution was injected into the autoclave with a syringe under CO2 atmosphere, followed by the addition of CHO. The autoclave was heated to a desired temperature in an oil bath and the 1.5 MPa pressure of CO2. The mixture was stirred for the allotted reaction time, then quickly cooled to room temperature and vented in a fume hood. A small aliquot of the resultant copolymerization mixture was removed from the reactor for 1H NMR analysis. The remaining copolymerization mixture was then dissolved in chloroform, quenched with 5 % HCl solution in methanol, and precipitated from methanol. The copolymer was collected and dried in vacuum to constant weight, and the copolymer yield was determined. The carbonate linkages were determined from the relative intensity of the cyclohexyl methine proton signals (4.6 ppm for carbonate linkages and 3.4 ppm for ether linkages) in the 1H NMR spectrum of the polymer. The molar mass and the molar mass distribution of the resulting polymer were determined by GPC.
Results and discussion
Syntheses of complexes
Substituted derivatives of the parent ligand salen were prepared by condensation of salicylaldehyde derivatives with ethylenediamine. Substituted salicylaldehydes were prepared via the Reimer–Tiemann reaction or through lithium aluminum hydride reduction followed by manganese dioxide oxidation of a substituted salicylic acid [37–39]. Stirring the Schiff base ligands with cobaltII acetate tetrahydrate under aerobic conditions afforded the requisite CoIII complexes [38]. The nature of the R1, R2, and R3 substituents, the hydrocarbonated chain R, and their abbreviations are also shown in Fig. 1.
The product was sufficiently dried under reduced pressure. Complexes 1–6 were isolated as a solid powder. Numerous attempts to isolate complexes 1–5 in a mixture of methylene chloride and hexane or other solvents were proved to be unsuccessful. Fortunately, the structure of a closely related complex 6 could be elucidated by single crystal X-ray analysis. Crystal suitable for X-ray diffraction was grown from a mixture of methylene chloride and hexane at 20 °C. A single crystal was selected for X-ray analysis (Table 1). As shown in Fig. 2, the structure is indeed a six-coordinate octahedral as the structure of the salen CoIII(2,4-dinitrophenoxy) [35, 36]. X-ray diffraction data showed that the crystal was analogous monomeric structures. The overall geometry around the metal cobalt was a six-coordinate octahedral with an average compressed axial O(2)–Co(1)–O(3) bond angle of 167.20(9)°, and equatorial O(1)–Co(1)–N(1), N(1)–Co(1)–N(2), O(2)–Co(1)–O(4), and O(4)–Co(1)–O(1) bond angles of 91.22(9)°, 92.17(10)°, 100.69(9)°, and 86.57(9)°, respectively. The distances from the Co atom to O(1), O(2), O(3), O(4), N(1), and N(2) were 1.871(2), 1.871(2), 1.962(2), 1.988(2), 1.880(2), and 1.899(3) Å, respectively (Table 2). The average Co–N and Co–O bond lengths are in the same range as corresponding values in similar octahedral CoIII systems [40, 41]. The molecular structure of complex 6 is shown in Fig. 2.
Copolymerization of CHO and CO2
Different CoIII complexes were evaluated as catalysts for the copolymerization of CHO and CO2 in the presence of [PPN]Cl under various reaction conditions. These complexes exhibit different catalytic activities. As shown in Table 3, complex 1 bearing ligand with the R1 being a donor electronic methyl group displays the highest catalytic activity (see entry 1, in Table 3). The reason that the catalytic activity depended on the structure of ligand should be attributed to that donor electronic methyl could stabilize the active salen CoIII species against decomposition to inactive salen CoII by reversibly intramolecular Co–O bond formation and dissociation. When the R2 or R3 group is replaced by an iodine atom, complexes 3 and 5 present a moderate catalytic activity (see entries 3 and 5, in Table 3). Complex 6 bearing the sterically less bulky ligands shows the lowest activity among its analogues complexes 1–6. Under the same conditions, when the R1 group is replaced by a hydrogen atom, complex 2 exhibits a lower catalytic activity (see entry 2, in Table 3). While diimine-bridges with the two carbon atoms are superseded by that with the three carbon atoms, the catalytic activity is dramatically decreased. The catalytic activity is influenced markedly by the nature of the substituents grafted on the ligands molecules and the length of the diimine bridges between the two nitrogen atoms in the ligands, respectively. These results might be mainly ascribed to the steric bulk effect of the substituents R on the phenolates of the ligand. This agrees well with the literature data [5]. These differences in ligands framework and the length of the diimine bridges between the two nitrogen atoms in the ligands also reflect on the selectivity formation of poly(cyclohexenylene carbonate) (PCHC) over cyclohexene carbonate (CHC) and the resulting copolymer number average molecular weights (M n ). Complex 1 displays the highest PCHC/CHC selectivity and yields polymer with the highest M n . However, complex 6 presents the lowest PCHC/CHC selectivity and produces polymer with the lowest M n . Both the length of the diimine bridges and the substituents on the ligands do not have a clear effect on the microstructure of the resultant polymer. With complexes 1–6 the copolymerization afford products possessing >99 % carbonate linkages.
The mole ratio of [PPN]Cl to the complex 1 played an important role in the copolymerization of CHO with CO2, as listed in Table 4 (entries 1–4). Although almost negligible yield was observed in the absence of cocatalyst (see entry 1 in Table 4), the addition of cocatalyst [PPN]Cl resulted in the production of the alternating copolymer (see entries 2–4, in Table 4). Optimization of the molar ratio between complex 1 and [PPN]Cl revealed that the highest yield was achieved using 1 equiv. of [PPN]Cl. However, the increase in the ratio of [PPN]Cl to complex 1 above 2 equiv. could increase the reaction activities. The CHC only was obtained. The CHC formation might be based on the presence of a depolymerization reaction that is promoted at higher [PPN]Cl concentrations. It is most likely due to a backbiting reaction starting from an alcoholate chain end that is displaced from the CoIII center in a coordination equilibrium with [PPN]Cl after epoxide ring opening [18].
The influence of reaction temperature on the copolymerization of CHO and CO2 was investigated using complex 1/[PPN]Cl (see entries 3 and 5–7, in Table 4). Dramatic increase in catalytic activity with temperature was observed, and the turnover frequency (TOF) at 80 °C (48.7 h−1) almost doubles that at 60 °C (25.6 h−1) (see entries 3 and 5, in Table 4), which reaches to 52.2 h−1 when the copolymerization is performed at 100 °C (see entry 6, in Table 4). The highest catalytic activity (54.5 h−1) is achieved at 120 °C (see Entry 7, in Table 4). While the yield of copolymer increases with increasing temperature up to about 80 °C, and then decreases with the further increase. Similar results have been observed previously [33]. In agreement with the increase in yield, M n of the obtained copolymer increases correspondingly from 21.6 kg mol−1 at 60 °C to 38.7 kg mol−1 at 80 °C. In the meantime, polymer dispersity index remains narrow and almost constant (PDI = 1.2). It is generally known that the homopolymerization of CHO easily takes place at elevated temperatures and is normally observed at catalyst systems with regard to much-studied zinc complexes [10]. Moreover, the polycarbonates obtained at the range of 60–120 °C all have >99 % carbonate linkages.
Obviously, the mole ratio of monomer to complex plays an important role on the TOF of copolymerization, as listed in Table 4 (entries 3 and 8–10). For example, in the experiment of entry 3, TOF (10 h) is 48.7 h−1 when 1,000 equiv. of CHO are used. As the ratio of CHO to complex 1 decreases to 200 equiv. in entry 8, the reaction activity decreases by almost thrice, compared with entry 3. Meanwhile, PDI also slightly decreases from 1.2 to 1.1, and M n decreases significantly from 38.7 to 14.9 kg mol−1. However, the further increases in the ratio of CHO to complex 1 above 1,000 equiv. could reduce the reaction activities (see entries 3 and 10, in Table 3). Especially, when over 1,000 equiv. of CHO are selected, both drops in catalytic activity and yield of the resultant product are observed (entry 10), which could be attributed to the lowing of the catalytic concentration when overloading monomer.
Besides operation temperature and ratio of monomer to complex, initial CHO concentration is another important issue for the reaction. In our results, it is observed that the catalytic activity increases as the initial CHO concentration increases from 30 to 50 mol l−1 (see entries 3, 11, and 12, in Table 4) and decreases then with the further increase in CHO concentration (entry 13 in Table 4). The prolonged reaction does not lead to an observable decrease in selectivity for PCHC formation, which can be explained by the so-called solvent effect. A similar solvent effect was reported for the copolymerization with binary catalyst systems [32, 42].
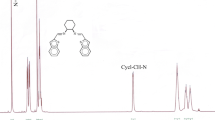
13C NMR spectroscopy has been extensively used to investigate CHO incorporation into polymer backbones, which can be used to estimate the tacticity of the copolymer [2, 14]. Figure 3 presents a 13C NMR spectrum of the PCHC (entry 3 in Table 4). The spectrum is very similar to those of PCHC prepared with (salen)CoX catalysts [5]. Clearly, the carbonyl regions at δ = 153.80–153.66 and 153.25–153.30 ppm can be assigned to the isotactic and syndiotactic structures of the PCHC chain, respectively [2]. The peaks at 153.25–153.30 ppm are assigned to the central carbonyl carbons of r-centered tetrads, whereas the peaks at 153.80–153.66 ppm are attributed to the central carbonyl carbons of m-centered tetrads [2, 14]. PCHCs revealed a strong contribution correlating to r-centered tetrads (78 %), indicative of syndiotactic PCHC.
Carbonyl region of the 13C NMR spectrum (CDCl3, 125 MHz) of PCHC from the copolymerization of CO2/CHO sample (entry 2 in Table 4)
MALDI-TOF mass spectrometry is specifically suitable for structural and end-group analysis [5]. Figure 4 displays a spectrum of the resultant PCHC obtained (entry 3 in Table 4). At least two series of signals with regular repeat intervals (repeating units of 142.2 mass units) were observed in the mass spectra. For the first series of peaks at the m/z of 1,931.6, 2,073.8, 2,216.0, 2,358.2, 2,500.4, and 2,642.6, there was a difference of 142.2 in m/z between every two neighboring peaks, which was also seen in the second series at the m/z of 1,947.7, 2,089.9, 2,232.1, 2,374.3, 2,516.5, and 2,658.7. Moreover, in the second series peaks in m/z were correspondingly 16 larger than those in the first series. Additionally, the weight number of propylene carbonate unit is 142.2. Thus, it could be known by calculation that the first series peaks resulted from the copolymerization reaction was initiated by insertion of CHO into a Co-acetate bond of the catalytic species attached to a Co center, and terminated with acetate groups and Na+ ions, and K+ ions instead of Na+ ions for the second series.
MALDI-TOF mass spectrum of PCHC from the alternating copolymerization of CO2/CHO sample (entry 2 in Table 4). \( \mathrm{mass}\left( {{m \left/ {z} \right.}} \right)={M_{{\mathrm{C}{{\mathrm{H}}_3}\mathrm{COO}}}}+n{M_{\mathrm{repeating}}}+{M_{{\mathrm{N}{{\mathrm{a}}^{+}}}}} \)or\( \mathrm{mass}\left( {{m \left/ {z} \right.}} \right)={M_{{\mathrm{C}{{\mathrm{H}}_3}\mathrm{COO}}}}+n{M_{\mathrm{repeating}}}+{M_{{{{\mathrm{K}}^{+}}}}} \) (where \( {M_{{\mathrm{C}{{\mathrm{H}}_3}\mathrm{OO}}}}=59.0 \), \( {M_{\mathrm{repeating}}}=142.2 \) \( {M_{{\mathrm{N}{{\mathrm{a}}^{+}}}}}=23 \), \( {M_{{{{\mathrm{K}}^{+}}}}}=39 \)
On the basis of the information presented so far, some proposed mechanism illustrated that the monomer was inserted to the cobalt–oxygen bond of the propagating end, as shown in Scheme 1. [PPN]Cl coordinated to the metal center in the axial site transferred to the propagating metal–polymer chain, thereby labilizing the metal alkoxide bond and facilitating the insertion of CO2 [3, 6, 17, 18, 21, 24]. The CHO monomer might be first favorably inserted into the Co–OR bond of the Schiff base cobalt complex, followed by the insertion of the CO2 monomer. Subsequent alternating copolymerization of CHO and CO2 affords the alternating repeating unit structure to yield the polycarbonate. The side reaction to yield the cyclic carbonate could take place through the backbiting degradation of the growing polymer–catalyst complex, which may lead to the lower polymer yield. A mechanism similar to that proposed by Darensbourg and Yarbrough using chromium salen and N-MeIm in the copolymerization of CO2 and cyclohexene oxide [14].
Copolymerization of PO and CO2
By use of complex 1 and [PPN]Cl catalyst system, the copolymerization of CO2 and PO ([PO]0/[[PPN]Cl]0/[complex 1]0 = 500/1/1) selectively gave the alternating copolymer in 1,4-dioxane at 60 °C in 24 h (carbonate linkages = 99 %, polycarbonate/cyclic carbonate = 99/1, M n = 6,400, M w/M n = 1.21; Table 5, run 2), although the reaction proceeded rather slowly (36.8 % yield). Also at 80 °C, the copolymeriation proceeded more rapidly to attain 42.1 % polymer yield in the same reaction time, slightly lowering the selectivity (carbonate linkages = 95.8 %, polycarbonate/cyclic carbonate = 92/8, M n = 7,800, M w/M n = 1.31; Table 5, run 3). When the copolymerization was conducted at an elevated temperature, such as 100 °C, a significant amount of cyclic carbonate (polycarbonate/cyclic carbonate = 30/70) was generated under otherwise similar conditions (Table 5, run 3).
The high selectivity of polycarbonate over cyclic carbonate at 60 °C was maintained after a longer reaction time to attain quantitative formation of the alternating copolymer. The alternating copolymeriation of CO2 and PO by using complex 1 and [PPN]Cl catalyst system was carried out at 60 °C for 48 h and afforded the alternating copolymer almost quantitatively (polycarbonate/ cyclic carbonate = 93/7, carbonate linkages = 99 %, M n = 13,900, M w/M n = 1.26, Table 5, run 5).
Conclusions
In summary, a series of cobalt/Schiff base complexes 1–6 were investigated for the alternating copolymerization of CO2 with epoxide (CHO and PO) to afford completely alternating polycarbonates with good activity in presence of [PPN]Cl. The resultant polymer has high molecular weight and high carbonate linkages as well as narrow molar mass distribution. The molecular weight of the copolymers is also sensitive to the concentration of CHO in the feed for the complexes. In addition, the solid-state structure of complex 6 was characterized, providing valuable information about the mechanism of the catalytic polymerization reaction. This strategy allows considerable scope for ligand modification and thus might lead to the design of new, efficient, and practical catalysts for the copolymerization of epoxides with CO2.
References
Darensbourg DJ (2007) Chem Rev 107:2388–2410
Nakano K, Nozaki K, Hiyama T (2001) Macromolecules 34:6325–6332
Nozaki K, Nakano K, Hiyama T (1999) J Am Chem Soc 121:11008–11009
Koinuma H (2007) React Funct Polym 67:1129–1136
Cohen CT, Thomas CM, Peretti KL, Lobkovsky EB, Coates GW (2006) Dalton Trans 1:237–249
Nakano K, Kobayashi K, Nozaki K (2011) J Am Chem Soc 133:10720–10723
Super M, Berluche E, Costello C, Beckman E (1997) Macromolecules 30:368–372
Sugimoto H, Ogawa A (2007) React Funct Polym 67:1277–1283
Atsushi O (2010) Chem Lett 39:1066–1068
Kim I, Kim SM, Ha CS, Park DW (2004) Macromol Rapid Commun 25:888–893
Mang S, Cooper AIM, Colclough E, Chauhan N, Holmes AB (2000) Macromolecules 33:303–308
Cheng M, Moore DR, Reczek JJ, Chamberlain BM, Lobkovsky EB, Coates GW (2001) J Am Chem Soc 123:8738–8749
Moore DR, Cheng M, Lobkovsky EB, Coates GW (2003) J Am Chem Soc 125:11911–11924
Darensbourg DJ, Yarbrough JC (2002) J Am Chem Soc 124:6335–6342
Paddock RL, Nguyen ST (2001) J Am Chem Soc 123:11498–11499
Darensbourg DJ, Mackiewicz RM, Rodgers JL, Fang CC, Billodeaux DB, Reibenspies JH (2004) Inorg Chem 43:6024–6034
Nakano K, Nakamura M, Nozaki K (2009) Macromolecules 42:6972–6980
Darensbourg DJ, Ulusoy M, Karroonnirum O, Poland RR, Reibenspies JH, Cetinkaya B (2009) Macromolecules 42:6992–6998
Shi L, Lu XB, Zhang R, Peng XJ, Zhang CQ, Li JF, Peng XM (2006) Macromolecules 39:5679–5685
Liu BY, Gao YH, Zhao X, Yan WD, Wang XH (2010) J Polym Sci Part A: Polym Chem 48:359–365
Rao DY, Li B, Zhang R, Wang H, Lu XB (2009) Inorg Chem 48:2830–2836
Seong JE, Na SJ, Cyriac A, Kim BW, Lee BY (2010) Macromolecules 43:903–908
Ren WM, Zhang X, Liu Y, Li JF, Wang H, Lu XB (2010) Macromolecules 43:1396–1402
Noh EK, Na SJ, Kim SW, Lee BY (2007) J Am Chem Soc 129:8082–8083
Lu XB, Darensbourg D (2012) J Chem Soc Rev 41:1462–1484
Lu XB, Wang Y (2004) Angew Chem Int Ed 43:3574–3577
Niu YS, Zhang WX, Li HC, Chen XS, Sun JR, Zhuang XL, Jing XB (2009) Polymer 50:441–446
Qin Z, Thomas CM, Lee S, Coates GW (2003) Angew Chem Int Ed Engl 42:5484–5487
Nakano K, Kamada T, Nozaki K (2006) Angew Chem Int Ed 45:7274–7277
Cohen CT, Chu T, Coates GW (2005) J Am Chem Soc 127:10869–10878
Lu XB, Lei S, Wang YM, Zhang R, Zhang YJ, Peng XJ, Zhang ZC, Li B (2006) J Am Chem Soc 128:1664–1674
Cohen CT, Coates GW (2006) J Polym Sci A Polym Chem 44:5182–5191
Niu YS, Zhang WX, Pan X, Chen XS, Zhuang XL, Jing XB (2007) J Polym Sci A Polym Chem 45:5050–5056
Paddock RL, Hiyama Y, McKay JM, Nguyen ST (2004) Tetrahedron Lett 45:2023–2026
Niu YS, Li HC, Chen XS, Zhang WX, Zhuang XL, Jing XB (2009) Macromol Chem Phys 210:1224–1229
Zhuang XL, Oyaizu K, Niu YS, Koshika K, Chen XS, Nishide H (2010) Macromol Chem Phys 211:669–679
Postmus CJ, Kaye IA, Craig CA, Matthews RS (1964) J Org Chem 29:2693–2698
Ren WM, Liu Y, Wu GP, Liu J, Lu XB (2011) J Polym Sci A Polym Chem 49:4894–4901
Wisniewski M, Le BA, Spassky N (1997) Macromol Chem Phys 198:1227–1238
Ware DC, Denny WA, Clark GR (1997) Acta Crystallogr Sect C 53:1058–1059
Weiss MC, Bursten BB, Peng SM, Goedken VL (1976) J Am Chem Soc 98:8021–8031
Sugimoto H, Ohtsuka H, Inoue S (2005) J Polym Sci A Polym Chem 43:4172–4186
Acknowledgment
Gratitude is expressed to the Young Scientists Fund of the National Natural Science Foundation of China (grant no. 51003051), Science-Technology Foundation for Middle-Aged and Young Scientist of Shandong Province, China (grant no. BS2011CL021), and the High-level Talent Initial Funding for Scientific Research of Qingdao Agricultural University (grant no. 630924) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Niu, Y., Li, H. Alternating copolymerization of carbon dioxide and cyclohexene oxide catalyzed by salen CoIII(acetate) complexes. Colloid Polym Sci 291, 2181–2189 (2013). https://doi.org/10.1007/s00396-013-2957-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-013-2957-2