Abstract
Drying dissipative patterns of de-ionized suspensions (colloidal crystal-state at high concentrations) of the thermosensitive gels of poly (N-isopropylacrylamide) with various sizes (ca. 400–1,500 nm in diameter at 20 °C) were observed at 20 and 45 °C on a cover glass, a watch glass, and a Petri glass dish. The broad rings were observed and their size decreased as gel concentration decreased. Formation of the monodispersed agglomerated particles and their ordered arrays were observed irrespective of gel size. The macroscopic flickering spoke-like patterns were observed for the gel spheres from 70 to 600 nm in diameter at 20 °C, but almost disappeared for extremely large spheres, poly(N-isopropylacrylamide)(1500-5). This work clarified the formation of the drying microscopic structures of (a) ordered rings, (b) flickering ordered spoke lines, (c) net structure, and (d) lattice-like ordered structures of the agglomerated particles. The ordered rings became rather vague as gel size increased. The large net structures formed so often for large gels. Size effect on the lattice patterns was not recognized so clearly. The role of the electrical double layers around the agglomerated particles and the interaction of the particles with the substrate surfaces during dryness are important for the ordering. The microscopic drying patterns of gel spheres were quite different from those of linear type polymers and also from typical colloidal hard spheres, though the macroscopic patterns such as broad ring formation at the edges of the dried film were similar to each other.







Similar content being viewed by others
References
Okubo T (2006) Molecular and colloidal electro-optics. SP Stoylov, MV Stoimenova (eds). Taylor & Francis: New York, p 573
Okubo T (2008) Nanoparticles: syntheses, stabilization, passivation and functionalization. Nagarajan R, Hatton TA (eds). ACS Book: Washington DC, p 256
Okubo T (2010) Macromol Symp 288:67
Gribbin G (1999) Almost everyone’s guide to science. The universe, life and everything. Yale University Press, New Haven
Ball P (1999) The self-made tapestry. Pattern formation in nature. Oxford Univ Press, Oxford
Terada T, Yamamoto R, Watanabe T (1934) Sci Paper Inst Phys Chem Res Jpn 27:173, Proc Imper Acad Tokyo 10:10
Terada T, Yamamoto R, Watanabe T (1934) Sci Paper Inst Phys Chem Res Jpn 27:75
Nakaya U (1947) Memoirs of Torahiko Terada (Japanese). Kobunsya, Tokyo
Okubo T, Kimura H, Kimura T, Hayakawa F, Shibata T, Kimura K (2005) Colloid Polym Sci 283:1
Okubo T (2006) Colloid Polym Sci 285:225
Okubo T (2009) Colloid Polym Sci 287:167
Okubo T, Okamoto J, Tsuchida A (2009) Colloid Polym Sci 287:351
Okubo T (2009) Colloid Polym Sci 287:645
Palmer HJ (1976) J Fluid Mech 75:487
Anderson DM, Davis SH (1995) Phys Fluids 7:248
Pouth AF, Russel WB (1998) AIChEJ 44:2088
Burelbach JP, Bankoff SG (1998) J Fluid Mech 195:463
Fischer BJ (2002) Langmuir 18:60
Okubo T (2006) Colloid Polym Sci 284:1191
Okubo T (2006) Colloid Polym Sci 284:1395
Okubo T, Okamoto J, Tsuchida A (2007) Colloid Polym Sci 285:967
Okubo T (2007) Colloid Polym Sci 285:1495
Okubo T, Okamoto J, Tsuchida A (2008) Colloid Polym Sci 286:385
Okubo T, Okamoto J, Tsuchida A (2008) Colloid Polym Sci 286:941
Yamaguchi T, Kimura K, Tsuchida A, Okubo T, Matsumoto M (2005) Colloid Polym Sci 283:1123
Okubo T (2006) Colloid Polym Sci 285:331
Vanderhoff JW (1973) J Polym Sci Symp 41:155
Nicolis G, Prigogine I (1977) Self-organization in non-equilibrium systems. Wiley, New York
Ohara PC, Heath JR, Gelbart WM (1997) Angew Chem 109:1120
Deegan RD, Bakajin O, Dupont TF, Huber G, Nagel SR, Witten TA (1997) Nature 389:827
Maenosono S, Dushkin CD, Saita S, Yamaguchi Y (1999) Langmuir 15:957
Deegan RD, Bakajin O, Dupont TF, Huber G, Nagel SR, Witten TA (2000) Phys Rev E 62:756
Nikoobakht B, Wang ZL, El-Sayed MA (2000) J Phys Chem 104:8635
Ung T, Litz-Marzan LM, Mulvaney P (2001) J Phys Chem B 105:3441
Okubo T, Okamoto J, Tsuchida A (2010) Colloid Polym Sci 288:189
Okubo T, Kanayama S, Ogawa H, Hibino M, Kimura K (2004) Colloid Polym Sci 282:230
Okubo T, Okamoto J, Takahashi S, Tsuchida A (2009) Colloid Polym Sci 287:933
Okubo T, Hagiwara A, Kitano H, Okamoto J, Takahashi S, Tsuchida A (2009) Colloid Polym Sci 287:1155
Okubo T, Okamoto J, Tsuchida A (2010) Colloid Polym Sci 288:981
Okubo T, Takahashi S, Tsuchida A, Colloid Polym Sci 289:1729
Okubo T, Mizutani M, Takahashi S, Tsuchida A (2010) Colloid Polym Sci 288:1551
Okubo T (2011) Colloid Polym Sci 289:159
Okubo T (2011) Colloid Polym Sci 289:1205
Okubo T, Mizutani M, Takahashi S, Tsuchida A (2010) Colloid Polym Sci 288:1435
Okubo T, Takahashi S, Tsuchida A (2011) Colloids Surfaces B 87:11
Okubo T (2011) Colloids Surfaces B 87:439
Okubo T, Itoh E, Tsuchida A, Kokufuta E (2006) Colloid Polym Sci 285:339
Okubo T, Suzuki D, Tsuchida A (2012) Colloid Polym Sci 290:867
Okubo T, Suzuki D, Yamagata T, Katsuno A, Mizutani M, Kimura H, Tsuchida A (2011) Colloid Polym Sci 289:807
Okubo T, Suzuki D, Tsuchida A (2012) Colloid Polym Sci 290:411
Okubo T, Suzuki D, Yamagata T, Katsuno A, Sakurai M, Kimura H, Tsuchida A (2011) Colloid Polym Sci 289:291
Okubo T, Suzuki D, Yamagata T, Horigome K, Shibata K, Tsuchida A (2011) Colloid Polym Sci 289:1273
Suzuki D, Horigome K, Yamagata T, Shibata K, Tsuchida A, Okubo T (2011) Colloid Polym Sci 289:1799
Suzuki D, Yamagata T, Horigome K, Shibata K, Tsuchida A, Okubo T (2011) Colloid Polym Sci 290:107
Holtz JH, Asher SA (1997) Nature 389:829
Pelton R (2000) Adv Colloid Interf Sci 85:1
Xia Y, Cates B, Yin Y, Lu Y (2000) Adv Mater 12:693
Hellweg T, Dewhurst CD, Bruckner E, Kratz K, Eimer W (2000) Colloid Polym Sci 278:972
Debord JD, Lyon LA (2000) J Phys Chem B 104:6330
Xia Y (2001) Adv Mater 13:369
Gao J, Hu Z (2002) Langmuir 18:1360
Stoeber W, Fink A, Bohn E (1968) J Colloid Interf Sci 26:62
Okubo T, Hongyo K, Enokida A (2087) (1984) J Chem Soc. Faraday Trans 1 80:2087
Acknowledgments
Financial supports to TO from the Ministry of Education, Culture, Sports, Science and Technology, Japan for Exploratory Research and those for Scientific Research (B) to TO, DS, and AT from Japan Society for the Promotion of Science are greatly acknowledged. DS also acknowledges Grand-in-Aid for Young Scientists (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (22685024). The research funds from AMX Co. (Tokyo) to TO are appreciated deeply. The authors thank Tomoyo Yamagata and Koji Horigome for their help in preparing the gel spheres.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
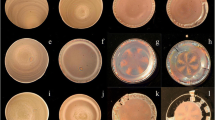
Fig. S1. Drying patterns of pNIPAm(400-5) (A), pNIPAm(600-5) (B) and pNIPAm(1500-5) spheres (C) on a cover glass {a–e (a, c), a–d (b), 0.1 ml}, a watch glass {f–j (a, c), e–h (b), 0.7 ml} and a glass dish {k–o (a, c), i–l (b), 0.7 ml} at 20 °C. Humidity = 35 %; a, f, k (a) 3.89 wt%; a, e, i (b) 3.88 wt%; a, f, k (c) 3.10 wt%; b, g, l (a) 0.97 wt%; b, f, j (b) 1.16 wt%; b, g, l (c) 0.78 wt%; c, h, m (a) 0.195 wt%; c, g, k (b) 0.194 wt%; c, h, m (c) 0.155 wt%; d, i, n (a) 0.052 wt%; d, h, l (b) 0.078 wt%; d, i, n (c) 0.0413 wt%; e, j, o (a) 0.0195 wt%; e, j, o (c) 0.0155 wt%
Fig. S2. Microscopic drying patterns of pNIPAm(400-5) (a), pNIPAm(600-5) (b) and pNIPAm(1500-5) (c) on a cover glass (a–d, 0.1 ml) a watch glass (e–h, 0.7 ml) and a glass dish (i–p, 0.7 ml) at 20 °C. Humidity = 35 %, a–h 0.97 wt% (a), 3.88 wt% (b), 0.78 wt% (c), i-l 3.89 wt% (a), 3.88 wt% (b), 3.10 wt% (c), m–p 0.97 wt% (a), 1.16 wt% (b), 0.78 wt% (c), a–d (a–c) are the pictures from the left edge to the right; e, i, m to h, l, p are from the center to the right edge, full scale is 100 μm
Fig. S3. Microscopic patterns of pNIPAm(400-5) (a), pNIPAm(600-5) (b) and pNIPAm(1500-5) gels (c) at 45 and 20 °C. Circles ordered ring, triangles ordered spoke-line, crosses net, squares lattice
ESM 1
PPT 1,978 kb
Rights and permissions
About this article
Cite this article
Okubo, T., Suzuki, D. & Tsuchida, A. Drying dissipative structures of thermosensitive gel spheres of poly (N-isopropylacrylamide). Influence of gel size. Colloid Polym Sci 290, 1901–1911 (2012). https://doi.org/10.1007/s00396-012-2727-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-012-2727-6