Abstract
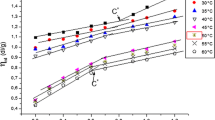
Interactions between anionic polyelectrolyte, poly(acrylic acid) (PAA), and cationic surfactant, alkyltrimethylammonium bromide (C n TAB), were investigated by rheological measurements in semidilute PAA solution. The dependences of the rheological behavior on the chain length of the surfactant, PAA neutralization degree, and temperature were discussed. The results revealed that both dodecyl and cetyltrimethylammonium bromides (C12TAB and C16TAB) could increase the viscosity of PAA solution when the surfactant amounts surpassed a critical surfactant concentration (C c), and C c of C16TAB was lower than that of C12TAB at same PAA neutralization degree. The increase of viscosity is attributed to the surfactant micelles bridging of the polymer chains and confine the mobility PAA chain. On the other hand, it is found that the hydrogen bonding also played an important role in the PAA–C n TAB system, especially in lower neutralization degree PAA solution, which results in the viscosity increase rapidly with the added surfactant into lower neutralization degree PAA solution.







Similar content being viewed by others
References
Holmberg K, Jonsson B, Kronberg B, Bo L (2002) Surfactants and polymers in aqueous solution. Wiley, West Sussex, England
Jain N, Trabelsi S, Guillot S, McLoughlin D, Langevin D, Letellier P, Turmine M (2004) Langmuir 20:8496–8503
Colby RH, Plucktaveesak N, Bromberg L (2001) Langmuir 17:2937–2941
Hansson P (2001) Langmuir 17:4167–4180
Plucktaveesak N, Konop AJ, Colby RH (2003) J Phys Chem B 107:8166–8171
Merta J, Stenius P (1997) Colloid Surface A 122:243–255
Yan Y, Li L, Hoffmann H (2006) J Phys Chem B 110:1949–1954
Hansson P, Schneider S, Lindman B (2002) J Phys Chem B 106:9777–9793
Nause RG, Hoagland DA, Strey HH (2008) Macromolecules 41:4012–4019
Matsuda T, Annaka M (2008) Langmuir 24:5707–5713
Konop AJ, Colby RH (1999) Langmuir 15:58–65
Ritacco H, Kurlat D, Langevin D (2003) J Phys Chem B 107:9146–9158
Zhou SQ, Chu B (2000) Adv Mater 12:545–556
Hansson P, Almgren M (1994) Langmuir 10:2115–2124
Thünemann AF, Müller M, Dautzenberg H, Joanny J-F, Löwen H (2004) Adv Polym Sci 166:113–171
Fundin J, Hansson P, Brown W, Lidegran I (1997) Macromolecules 30:1118–1126
Lim PFC, Chee LY, Chen SB, Chen BH (2003) J Phys Chem B 107:6491–6496
Kiefer JJ, Somasundaran P, Ananthapadmanabhan KP (1993) Langmuir 9:1187–1192
Yoshida K, Dubin PL (1999) Colloid Surface A 147:161–167
Yoshida K, Sokhakian S, Dubin PL (1998) J Colloid Interf Sci 205:257–264
Wang C, Tam KC (2004) J Phys Chem B 108:8976–8982
Kong L, Cao M, Hai M (2007) J Chem Eng Data 52:721–726
Guillot S, McLoughlin D, Jain N, Delsanti M, Langevin D (2003) J Phys-condens Mat 15:S219–S224
Dobrynin AV, Colby RH, Rubinstein M (1995) Macromolecules 28:1859–1871
Dobrynin AV, Rubinstein M (2005) Prog Polym Sci 30:1049–1118
Wu Q, Du M, Shangguan Y, Zhou J, Zheng Q (2009) Colloid Surface A 332:13–18
Anghel DF, Saito S, Baran A, Iovescu A, Cornitescu M (2007) Colloid Polym Sci 285:771–779
Bakshi MS, Sachar S (2004) Colloid Polym Sci 282:993–999
Kogej K, Skerjanc J (1999) Langmuir 15:4251–4258
Wang C, Tam KC (2005) J Phys Chem B 109:5156–5161
Chakraborty T, Chakraborty I, Ghosh S (2006) Langmuir 22:9905–9913
Hansson P, Almgren M (1995) J Phys Chem 99:16684–16693
Trabelsi S, Raspaud E, Langevin D (2007) Langmuir 23:10053–10062
Proietti N, Amato ME, Masci G, Segre AL (2002) Macromolecules 35:4365–4372
Pryamitsyn V, Ganesan V (2006) J Rheol 50:655–683
Du M, Gong JH, Zheng Q (2004) Polymer 45:6725–6730
Lipatov YS, Shumsky VF, Getmanchuk IP, Gorbatenko AN (1982) Rheol Acta 21:270–279
Rubinstein M, Colby RH, Dobrynin AV (1994) Phys Rev Lett 73:2776–2779
Dou SC, Colby RH (2006) J Polym Sci Pol Phys 44:2001–2013
Boris DC, Colby RH (1998) Macromolecules 31:5746–5755
Hansson P, Almgren M (1996) J Phys Chem 100:9038–9046
Plucktaveesak N (2003) Solution rheology of polyelectrolytes and polyelectrolyte-surfactant systems. Doctor Dissertation, Pennsylvania State University
He Y, Zhu B, Inoue Y (2004) Prog Polym Sci 29:1021–1051
Acknowledgment
This work was supported by the Key Program of the National Natural Science Foundation of China (No. 50633030)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Q., Du, M., Ye, T. et al. Rheological behavior of PAA–C n TAB complex: influence of PAA charge density and surfactant tail length in PAA semidilute aqueous solution. Colloid Polym Sci 287, 911–918 (2009). https://doi.org/10.1007/s00396-009-2045-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-009-2045-9