Abstract
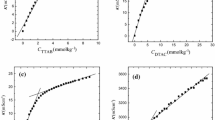
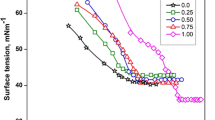
Different tetraalkylammonium, viz. N+(CH3)4, N+(C2H5)4, N+(C3H7)4, N+(C4H9)4 along with simple ammonium salts of bis (2-ethylhexyl) sulfosuccinic acid have been prepared by ion-exchange technique. The critical micelle concentration of surfactants with varied counterions have been determined by measuring surface tension and conductivity within the temperature range 283–313 K. Counterion ionization constant, α, and thermodynamic parameters for micellization process viz., \(\Delta G_m^{\text{0}} \), \(\Delta H_m^{\text{0}} \), and \(\Delta S_m^{\text{0}} \) and also the surface parameters, Γmax and Amin, in aqueous solution have been determined. Large negative \(\Delta G_m^{\text{0}} \)of micellization for all the above counterions supports the spontaneity of micellization. The value of standard free energy, \(\Delta G_m^{\text{0}} \), for different counterions followed the order \({\text{N}}^{\text{ + }} \left( {{\text{CH}}_{\text{3}} } \right)_4 >{\text{NH}}_{\text{4}}^{\text{ + }} >{\text{Na}}^{\text{ + }} >{\text{N}}^{\text{ + }} \left( {{\text{C}}_{\text{2}} {\text{H}}_5 } \right)_{\text{4}} {\text{ $>$ N}}^{\text{ + }} \left( {{\text{C}}_{\text{3}} {\text{H}}_{\text{7}} } \right)_4 >{\text{N}}^{\text{ + }} \left( {{\text{C}}_{\text{4}} {\text{H}}_{\text{9}} } \right)_4 \), at a given temperature. This result can be well explained in terms of bulkiness and nature of hydration of the counterion together with hydrophobic and electrostatic interactions.




Similar content being viewed by others

References
Yin H, Lei S, Zhu S, Huang J, Ye J (2006) Chem Eur J 12:2825
Paul A, Griffiths PC, Pettersson E, Stilbs P, Bales BL, Zana R, Heenan RK (2004) J Phys Chem B 108:3810
Griffiths PC, Paul A, Heenan RK, Penfold J, Ranganathan R, Bales BL (2005) J Phys Chem B 109:15775
Benrraou M, Bales BL, Zana R (2003) J Phys Chem B 107:13432
Shimizu S, Pires PAR, El Seoud OA (2004) Langmuir 20:9551
Pisárčik K, Devínsky F, Lacko I (2003) Acta Facult Pherm Univ Comenianae 50:119
Tiddy GJT (1980) Phys Rev 57:1
Khan A, Fontell K, Lindman B (1984) J Colloid Interface Sci 101:193
Lindman B, Puyal M-C, Kamenka N, Rymden R, Stilbs P (1984) J Phys Chem 88:5048
Mukerjee P (1967) Adv Colloid Interface Sci 1:241
Corkill JM, Goodman JF (1962) Trans Faraday Soc 58:206
Baumuller W, Hoffmann H, Ulbricht W, Tondre C, Zana R (1978) J Colloid Interface Sci 64:418
Wang Y, Dubin PL, Zang H (2001) Langmuir 17:1670
Oda R, Narayanan J, Hassan PA, Manohar C, Salkar RA, Kern F, Candau S (1998) Langmuir 14:4364
Soldi V, Keiper J, Romsted LS, Cuccovia IM, Chaimovich H (2000) Langmuir 16:59
Kawait T, Yasuda Y, Kon-no K (1995) Bull Chem Soc Jpn 68:2175
Eastoe J, Robenson BH, Heenan RK (1993) Langmuir 9:2820
Temsamani MB, Maeck M, Hassani IE, Hurwitz HD (1998) J Phys Chem B 102:3335
Acosta E, Bisceglia M, Fernandez JC (2000) Colloids Surf A 161:417
Moroni MA, Minardi RM, Schulz PC, Puig JE, Rodríguez JL (1998) Colloid Polym. Sci. 276:738
Stokkeland I, Skauge A, Høiland H (1987) J Soln Chem 16:45
Heuvelsland W, de Visser C, Somsen G (1978) J Phys Chem 82:29
Nakagaki M, Handa T (1984) ACS Symposium Series 253:73
Tanford C (1980) The Hydrophobic Effect: Formation of Micelles and Biological Membranes, vol. 2. Wiley, New York
Chakraborty A, Chakraborty S, Saha SK (2007) J Dispersion Sci Technol 28:984
La Mesa C (1990) J Phys Chem 94:323
Suarez MJ, Lopez-Fontan JL, Sarmiento F, Mosquera V (1999) Langmuir 15:5265
Myers D (1992) Surfactant science and technology. VCH, New York
Zana R (1996) Langmuir 12:1208
Mukhim T, Ismail K (2005) J Surface Sci Technol 21:113
Su TJ, Lu JR, Thomas RK, Penfold J (1997) J Phys Chem B 101:937
Weckström K, Hanu K, Rosenholm JB (1994) J Chem Soc, Faraday Trans 90:733
Morori A (1992) Micelles: Theoretical and applied aspects. Planum, New York
Evans DF, Ninham BW (1986) J Phys Chem 90:226
Bedo Z, Berecz E, Laktos I (1992) Colloid Polym Sci 270:799
González-Pérez A, Czapkiewicz J, Del Castillo JL, Rodríguez JR (2004) Colloid Polym Sci 282:1359
Galán JJ, González-Pérez A, Rodríguez JR (2003) J Therm Anal Cal 72:465
Shugihara G, Nakano TY, Sulthana SB, Rakshit AK (2001) J Oleo Sci 50:29
Umlong IM, Ismail K (2005) J Colloid Interface Sci 291:529
Rosen MJ, Cohen AW, Dahanayake M, Hua X (1982) J Phys Chem 86:541
Oh SG, Shah DO (1993) J Phys Chem 97:284
Carnero Ruiz C, Díaz-López L, Aguiar J (2007) J Colloid Interface Sci 305:293
Sulthana SB, Bhat SGT, Rakshit AK (1997) Langmuir 13:4562
Kang K, Kim H, Lim K (2000) Colloids Surfaces A 189:113
Acknowledgement
Financial support from the University Grants Commission, New Delhi, India, under Special Assistance Program (SAP, No. F/540/6/DRS/2002) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chakraborty, A., Saha, S.K. & Chakraborty, S. Effect of size of tetraalkylammonium counterions on the temperature dependent micellization of AOT in aqueous medium. Colloid Polym Sci 286, 927–934 (2008). https://doi.org/10.1007/s00396-008-1850-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-008-1850-x