Abstract
Stabilization and characterisation of water soluble colloidal MnO2 during the oxidation of sulphur-containing organic reductants “thiourea, thioactamide and methionine” by permanganate in aqueous neutral media are reported for the first time. Upon addition of permanganate to a solution of methionine, a transient species appears within the time of mixing, which is stable for several weeks. On the other hand, the transient species is unstable in the presence of thiourea and thioacetamide, respectively. The nature of manganese (IV) species present in the solution was characterized by spectrophotometric and coagulation measurements. On addition of HClO4, there is a decrease in the absorbance of the reaction mixture. Under pseudo first-order conditions ([reductants] > [\( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \)]), the reduction rate was very fast up to the formation of water soluble colloidal MnO2. The effect of various parameters, such as hydrogen ion concentration, amount of \( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \) and concentration of reductants were investigated. Mechanisms consistent with the observed results have been proposed and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reaction of oxygen-, nitrogen- and sulfur-containing reductants with manganese(VII) has been the subject of several investigators [1–5]. In some instances, the formation of manganese(IV) as an intermediate has been proposed [6–10]. In the permanganate oxidation of several organic reductants, the possible intermediate species are Mn(VI), Mn(V), Mn(IV) and Mn(III) [5, 11]. The Mn(II) species exhibits unique behaviour (auto catalyst) in many redox reactions of Mn(VII). The existence of different manganese species in aqueous solution and the tendency of Mn(II) to act as an autocatalyst give systems of considerable complexity. Therefore, different attempts have been made to confirm the intermediacy of manganese(IV) and manganese(III) by use of competitive experiments [6, 12–15].
The pioneering work of Perez-Benito et al. [16] suggested that the water soluble colloidal manganese dioxide can be prepared by potassium permanganate and sodium thiosulphate in aqueous neutral media (Eq. 1).
It has been established that the colloidal MnO2 sols acquire negative electrostatic charge in aqueous solutions and remain perfectly transparent for at least several years [12, 16, 17]. Water soluble colloidal MnO2 has the advantage over water-insoluble forms that conventional ultraviolet-visible (UV-Vis) spectrophotometer can be used to monitor their reactions with an inorganic and organic reductants. We have observed the formation and decomposition of water soluble colloidal MnO2 during the oxidation of paracetamol by permanganate spectrophotometrically at 420 nm [5].
Our preliminary observations indicate that addition of traces of sulphur-containing reductants (methionine, thiourea and thioacetamide) to a solution of permanganate enhances the absorbance of the reaction mixture at 420 nm significantly, and pink (λ max = 525 nm) reaction mixture becomes brown immediately, which is stable for some time. The present study was designed to determine the nature of the species formed in the reduction in permanganate by S-containing reductants. The details of \( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \) oxidation of methionine, thiourea and thioacetamide are not yet known; although the kinetics of thiourea oxidation by water soluble colloidal MnO2 has been reported earlier [18]. It was, therefore, thought of interest to investigate the reduction in Mn(VII) by such compounds with a view to having an insight into the formation of water soluble colloidal MnO2.
Experimental
The following analytical grade chemicals were used without further purification: methionine (Koch-light, Pures), thiourea (Sigma, Aldrich), thioacetamide (Fluka, Puriss) and potassium permanganate (Fluka, Puriss). Thiourea was recrystallised from warm 20% v/v aqueous ethanol before use. Perchloric acid, 20% (Fisher) was used to maintain the hydrogen ion constant. The solution of all the reagents were prepared in double-distilled, deionized and CO2-free water. The solution of permanganate was standardised by titration against oxalate and stored in black bottle. Thioacetamide was first dissolved in minimum quantity of acetic acid and then diluted to the desired concentration with deionized water. Spectrophotometer was used to monitor the process of reaction under different experimental conditions.
Product identification
In a typical experiment, methionine (=2.0 × 10−2 mol dm−3), potassium permanganate (=2.0 × 10−4 mol dm−3) and distilled water (30 cm3) were mixed at room temperature in a reaction flask. After 2 h, sodium bicarbonate (=0.4 mol dm−3) was added and stirred vigorously followed by dropwise addition of benzoyl chloride solution until precipitation was completed. N-benzoylmethionine sulphoxide was confirmed by the reported method [19]. Ammonia and carbon dioxide were not detected as the oxidation products of methionine.
The main oxidation product of thiourea was characterised as follows: solution of thiourea (=6.0 × 10−2 mol dm−3) was added to an acidic solution of permanganate (=6.0 × 10−4 mol dm−3). The reaction mixture turned drop pink to colourless, and 10 cm3 ethanol was added followed by concentrated HCl. After 1 h, white crystals were filtered and washed. The compound was identified by its infrared spectrum and was conformed as the dithiol bis (formamidinium) [20].
Results and discussion
The most interesting feature of the present observations is the fast change in the colour (pink to brown) of the permanganate in presence of methionine, thiourea and thioacetamide. The pink (λ max = 525) reaction mixture becomes brown immediately at room temperature. At lower thiourea and thioacetamide concentrations, the brown colour is stable for some time. On the other hand, the observed brown colour is stable for some days in presence of methionine.
Reduction of permanganate by methionine
It is well known that absorption spectra of permanganate solution possess a maximum absorption (λ max) at 525 nm (Fig. 1). When \( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \) (1.0 × 10−4 mol dm−3) is allowed to react with methionine at 25 °C, a readily distinguishable brown colour appears. To confirm the nature of the brown colour, the spectrum of reaction mixture was recorded at the end of the reaction (Fig. 1).The most characteristic part of manganese(VII) is the ligand to metal charge transfer transitions observable in the 350–650 nm region. Figure 1 shows that the spectra of \( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \)-methionine reaction product cover the whole visible region of the spectrum. These observations are in good agreement with the results of Perez-Benito et al. [16, 17] (Mn(IV) is commonly involved in the permanganate oxidation of organic reductants). The spectra of Mn(IV) species depend on the nature of reducing agent, acidity of the reaction mixture and absence and presence of complexing agents [11, 12].
Electronic absorption spectra of \( {\text{MnO}}^{ - }_{4} \) (filled circle) and the reaction product of \( {\text{MnO}}^{ - }_{4} \) and methionine reaction at 25 °C. Reaction conditions: \( {\text{MnO}}^{ - }_{4} \) (=1.0 × 10−4 mol dm−3); methionine (=0.6 × 10−4 mol dm−3; filled square) and 1.0 × 10−4 mol dm−3 (filled triangle)
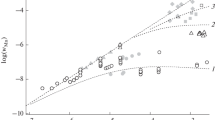
To confirm the nature of Mn(IV) species formed during the reduction in permanganate by methionine, the coagulation experiments were carried out and different electrolytes were used for this purpose. In presence of these electrolytes, the precipitation of manganese dioxide occurs, which suggests that the soluble Mn(IV) species is present in the form of colloidal particles of MnO2. The negative logarithms of the minimum electrolytes concentration required for the precipitation are plotted against cationic radius (Fig. 2). The fulfillment of the Beer–Lambert law by the resulting solution was also checked. The wavelength 400 nm was chosen to confirm the fulfillment of Beer–Lambert law by monitoring the absorbance of different solution of colloidal MnO2. The law is obeyed for the entire concentration range of methionine used in the present investigations. To obtain insight into the colloidal nature of MnO2, the Rayleigh’s law (absorbance = concentration/wavelength) was also used. The plot of log (absorbance) versus the log (wavelength) is linear (Fig. 3) [7, 9]. Thus, we may safely conclude that the available data are consistent with the formation of water soluble colloidal MnO2 as the intermediate during the reduction in \( {\text{MnO}}^{ - }_{4} \) by methionine.
Plots of log [electrolyte] versus cationic radius. Reaction conditions: \( {\text{MnO}}^{ - }_{4} \) (=1.0 × 10−4 mol dm−3); methionine (=1.0 × 10−4 mol dm−3); temperature = 25 °C; CoCl2 (1); MgCl2 (2); CaCl2 (3); BaCl2 (4); SrCl2 (5); MnCl2 (6); FeSO4 (7); NiCl2 (8); LiCl (9); NaCl (10); KCl (11) and NH4Cl (12)
Plots of log (absorbance) versus log (wavelength) for the product (colloidal MnO2) obtained from the reduction in \( {\text{MnO}}^{ - }_{4} \) by methionine (filled triangle), thiourea (filled circle) and thioacetamide (filled square). Reaction conditions: \( {\text{MnO}}^{ - }_{4} \) (=1.0 × 10−4 mol dm−3); methionine (=1.0 × 10−4 mol dm−3); thiourea (=0.4 × 10−4 mol dm−3); thioacetamide (=1.0 × 10−4 mol dm−3); temperature = 25 °C. Slopes = −6.8, −5.1 and −5.4 for methionine, thioacetamide and thiourea, respectively
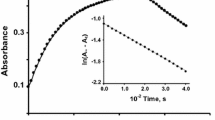
To see the effect of [methionine] on the formation of water soluble colloidal MnO2, a series of experiments were carried out at constant [\( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \)] (=1.0 × 10−4 mol dm−3) and temperature (=25 °C), and the reaction is monitored spectrophotometrically. These results are shown in (Fig. 4). At 400 nm, the absorbance increases until it reaches a maximum. The [methionine] needed to stabilise the colloidal MnO2 is (ca. 1.4 × 10−4 mol dm−3). Figure 4 clearly indicates the formation and stabilization of water soluble colloidal MnO2 during the reduction in permanganate by methionine.
To our knowledge, formation of soluble colloidal MnO2 has not been hitherto reported in the oxidation of amino acids by permanganate. It has been established that amino acids and sulphur-containing amino acids have two (-COOH and -NH2) and three (-SH, -COOH and -NH2) oxidation sites. To compare the reducing properties of these groups, some experiments were also performed with glycine under the same conditions of methionine. No brown colour was observed, even after prolonged incubation. On the basis of these observations and product identification (vide supra), we may safely conclude that -SH group of methionine has greater tendency to reduce manganese(VII) in comparison to -NH2 and -COOH groups. The sulphur is not as electronegative as oxygen and nitrogen, the C-S bond is less polar than the C-O and C-N bonds .Thus, a large partial negative charge resides on the oxygen and nitrogen atoms, activating it for protonation. This accounts for the non-availability of the electron pair at the seat of the reaction. Complete protonation of the carboxyl and amino groups lowers the reduction potential to such an extent that the oxidation site may effectively remain at the sulphur atom. Therefore, high reactivity of -SH group seems because of the presence of a lone pair of electron on S atom [18, 19, 21, 22].
From these data, the over all mechanism for the reaction can be represented by Scheme 1. Under the conditions of the experiment, zwitter ionic species of methionine is the major existing species.
Reduction of permanganate by thiourea and thioacetamide
Permanganate colour change from pink to brown was observed upon addition of thiourea (=1.0 × 10−4 mol dm−3) or thioacetamide (=1.0 × 10−4 mol dm−3) separately to the permanganate (=1.0 × 10−4 mol dm−3) within the time of mixing at room temperature. The UV-Vis spectral studies suggest that this brown colour is due to the formation of colloidal MnO2 as an intermediate. Spectra of \( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \) and the mixture containing thiourea and thioactamide immediately after mixing are shown in (Fig. 5). The spectrum of \( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \) (Fig. 1) is clearly different from those of \( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \)–thiourea (Fig. 5) and \( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \)–thioacetamide (Fig. 5) reaction product. Spectra of Fig. 5 are in closed agreement with the observations of other investigators. It is well known that if the brown colour is due to the formation of water soluble colloidal MnO2 as an intermediate, the spectrum will be mainly due to the scattering of light (Rayleigh’s law) [7, 9]. The plots of log (absorbance) versus log (wavelength) are linear (Fig. 3) with slopes ca. −5.4 and −5.1, respectively, for thiourea and thioacetamide.
Electronic absorption spectra of the reaction product of \( {\text{MnO}}^{ - }_{4} \)–thiourea (filled circle) and \( {\text{MnO}}^{ - }_{4} \)–thioacetamide (filled square) reduction at 25 °C. Reaction conditions: \( {\text{MnO}}^{ - }_{4} \) (=1.0 × 10−4 mol dm−3); thiourea (=1.0 × 10−4 mol dm−3); thioacetamide (=1.0 × 10−4 mol dm−3)
It is well established that the stability of colloidal MnO2 depends strongly on the pH of the reaction medium. The manganese(VII) changes to Mn(IV) in alkaline or weakly acid solution, while in strongly acid medium, the permanganate is further reduced forming Mn(II) as the final product .The colloidal MnO2 solution undergoes acid hydrolysis or is unstable [23] in aqueous solutions of [H+] > 1.0 × 10−3 mol dm−3. At 400 nm, the absorbance first increased until it reached a maximum then decreased with reductant concentrations. This behavior indicates the fast formation and slow disappearance of colloidal MnO2 during the course of reaction for both (thiourea and thioacetamide). It was noticed that the decomposition depends on the reaction conditions, i.e., [thiourea], [thioacetamide] and [HClO4]. The rate constants of the decomposition were determined under different experimental conditions and are summarised in Table 1. To gain further insight into the mechanistic aspects and the role of thiourea and thioacetamide concentrations, the effects of these [reductant] were studied at constant [\( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \)] (=1.0 × 10−4 mol dm−3) and temperature (=25 °C). The observed results are depicted graphically in Fig. 4 as an absorbance–[reductants] profile. As can be seen in Fig. 4 (typical example), as the [thiourea and thioacetamide] increases, the absorbance increases until it reaches a maximum then decreases with thiourea and thioacetamide concentration, respectively. On the other hand, at higher [thioacetamide] (≥2.0 × 10−4 mol dm−3), the formation and decomposition of colloidal MnO2 were not observed. The absorbance at 400 nm versus [thioacetamide] plot indicates the formation and decomposition of colloidal MnO2 (as an intermediate) taking place simultaneously in excess of [thioacetamide].
To gain further insight into the stability of colloidal MnO2, some kinetic experiments were carried out at constant [\( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \)], [methionine], [thiourea], [thioacetamide] and temperature. The observed results are summarised in Table 2. As can be seen from Table 2 (typical example), \( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \)–methionine reaction proceeds through the formation and stabilization of colloidal MnO2, whereas formation of MnO2 is not observed in thiourea– and thioacetamide–\( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \) reactions, respectively. It was noticed that the absorbance becomes 0.78 within the time of mixing and remains constant for ca. 20 min then decreased with time in presence of thiourea (Table 2). In presence of HClO4, decomposition of colloidal MnO2 is also observed for methionine–\( {\text{MnO}}^{{\text{ - }}}_{{\text{4}}} \) reaction (Table 2). The values of rate constants were calculated to be 35.8 mol−1dm3 s−1 (MnO2 formation for methionine) and 14.1 and 0.76 mol−1dm3 s−1 (MnO2 decomposition) for thiourea and thioacetamide, respectively.
In the redox reactions of manganese(VII) with organic reductants, medium of the reaction mixture plays an important role. The formation of Mn(IV) as an intermediate has been decided only in the weakly acidic solutions. In fact, very few chemical species are able to cause the reduction in MnO2 in neutral solutions [24]. Although a number of methods are available for the preparation of water soluble active species of MnO2, these are associated with some demerits [25, 26]. The formation of water soluble colloidal MnO2 has also been suggested on several occasions, but the reaction stops after the complete reduction in Mn(VII) to Mn(II). In this content, the sulphur-containing reductants (thiourea, thioacetamide and methionine), especially methionine, will get an edge over other reductants as water soluble colloidal MnO2 is stable for several days.
On the basis of the above results, the following mechanisms are proposed:
Ethylenediamine tetraacetic acid (EDTA; multifunctional α-amino acid) has a wide general application in analysis because of its powerful complexing action and commercial availability. Disodium salt of EDTA is capable of acting as quadridentate-, quinque dentate-, or six dentate ligand. In aqueous solution, there is always a competition between metal ions and hydrogen ions seeking the negative sites of EDTA. Therefore, to see the role of EDTA on the reduction in \( {\text{MnO}}^{{\text{ - }}}_{4} \) by methionine (formation of water soluble colloidal MnO2), the effect of EDTA was also studied in presence of methionine (1.0 × 10−4 mol dm−3) over fixed \( {\text{MnO}}^{ - }_{4} \) (1.0 × 10−4 mol dm−3) at 25 °C. As can be seen (Table 1), the reaction is sensitive even to small [EDTA]. The most interesting features of the present observations are the change of permanganate colour at 525 nm in the presence of EDTA and the vital role played by the order of mixing of methionine and EDTA in the formation of water soluble colloidal MnO2 .The experimental finding is that the pink reaction mixture containing \( {\text{MnO}}^{ - }_{4} \) (1.0 × 10−4 mol dm−3) and EDTA (1.0 × 10−3 mol dm−3) became colourless in the presence of methionine (1.0 × 10−4 mol dm−3; instead of dark brown, which is characteristic of water soluble colloidal MnO2 with λ max 400 nm). On the other hand, the end product of \( {\text{MnO}}^{ - }_{4} \)–methionine reaction was the colloidal MnO2 under our present experimental conditions (vide supra). Addition of EDTA to the solution resulted in a notable increase in reaction rate. Thus, we may safely conclude that water soluble colloidal MnO2 is not formed as a stable intermediate during the oxidation of methionine by permanganate in presence of EDTA.
The reactivity of the colloidal MnO2 with EDTA is compared with other compounds in Table 1. Interestingly, the reactivity of EDTA towards colloidal MnO2 is much higher than the corresponding oxidations of sulphur-containing reductants (thiourea, cysteine, glutathione and methionine). Table 1 shows that the reactivity decrease in the order EDTA > methionine > glutathione > cysteine > thiourea (caution: one should keep in mind the reaction conditions, method of preparation colloidal MnO2, temperature, etc). Although the data are not conclusive, it seems that -OH groups are responsible for the fast proton transfer from EDTA to MnO2. The dependence of reaction rate on [EDTA] has to be studied because possible information of interest about the EDTA–colloidal MnO2 interaction can be obtained.
Conclusion
The most interesting features of this study is the fast formation of water soluble colloidal MnO2 as an intermediate in the redox reactions of \( {\text{MnO}}^{ - }_{4} \) and sulphur-containing reductants. Reduction in Mn(VII) to Mn(IV) could probably be explained because of the higher reducing nature of sulphur. We are unaware of any precedence in the redox chemistry of these systems, especially regarding the formation of colloidal MnO2. The stabilisation of water soluble colloidal MnO2 in the oxidation of methionine is unique in the sense that Mn(II) was formed as final product of amino acid \( {\text{MnO}}^{ - }_{4} \) reaction. It is well established that complex formation result in considerable increase in the oxidation potential of a metal ion. The presence of -NH2 and -COOH groups in methionine may help to stabilise the water soluble colloidal MnO2 through complexation.
References
Hasan RM, Mousa MA, Wahdan MH (1988) J Chem Soc Daton Trans 3:605
Perez-Benito JF, Arias C, Brillas E (1990) Int J Chem Kinet 22:261
Arrizabalaga A, Andres-Ordax FJ, Fernandez-Aranguiz MY, Peche R (1997) Int J Chem Kinet 29:181
Raju Khan Z, Kabir-ud-Din (2005) Colloid Polym Sci 284:26
Kumar P, Khan Z (2006) Colloid Polym Sci 284:1155
Simandi LI, Jaky M (1976) J Am Chem Soc 98:1995
Mata-Perez F, Perez-Benito JF (1985) Can J Chem 63:988
Nagy A, Treindi L (1986) Nature 320:344
Freeman F, Kappos JC (1985) J Am Chem Soc 107:6628
Raju, Khan Z (2005) Bull Chem Soc Jpn 78:1218
Pimienta V, Lavabre D, Levy G, Micheau JC (1990) J Phys Chem 98:13294
Perez-Benito JF, Brillas E, Pouplana R (1989) Inorg Chem 28:390
Orban M, Lengyel I, Epstein IR (1991) J Am Chem Soc 113:1978
Perez-Benito JF, Arias C (1992) J Colloid Interface Sci 152:70
Jones TJ, Noyes RM (1983) J Phys Chem 87:4686
Perez-Benito JF, Arias C (1992) J Colloid Interface Sci 149:92
Perez-Benito JF, Arias C, Amat E (1996) J Colloid Interface Sci 177:288
Andrabi SMZ, Khan Z (2005) Colloid Polym Sci 284:36
Olatunji MA, Ayoko GA (1988) Polyhedron 7:11
Olatunji MA, McAuley A (1975) J Chem Soc Dalton P 682
Andrabi SMZ, Khan Z (2007) Colloid Polym Sci 285:389
Mehrotra M, Mehrotra RN (2003) J Chem Soc Dalton Trans P 3606
Khan Z, Kumar P, Kabir-ud-Din (2004) Colloids Surf A: Physicochem Eng Aspects 248:25
Perez-Benito JF (2003) Colloid Surf A Physicochem Eng aspects 225:145
Abd El-Salaam KM (1975) Z Phys Chem Nene Folge 95:139
Taniguchi S (1984) Bull Chem Soc Jpn 57:2683
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
AL-Thabaiti, S.A., Al-Nowaiser, F.M., Obaid, A.Y. et al. Formation, characterization and stabilization of water-soluble colloidal MnO2 in the oxidation of methionine, thiourea and thioacetamide by permanganate. Colloid Polym Sci 285, 1479–1485 (2007). https://doi.org/10.1007/s00396-007-1709-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-007-1709-6