Abstract
The main objective of this study is to prepare, thermally, sensitive microgel particles bearing thiol groups via precipitation polymerization of N-isopropylacrylamide (NIPAM), methylenebisacrylamide (MBA) and vinylbenzylisothiouronium chloride (VBIC) using 2-2′-azobis(2-amidinopropane)-dihydrochloride (V50) as initiator. The influence of various parameters has been investigated as a systematic study to point out the role of each reactant on polymerization conversion, and consequently, on particles and water-soluble polymer formation. The final microgel particles were characterized with respect to particle size and swelling ability. The aim of this paper is to complete our first short communication; Macromolecular symposia, 2000. 150: p. 283–290.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The preparation and the use of stimuli responsive materials have attracted the attention of various application areas. Regarding the elaboration of temperature-responsive microgels, the first work in this direction has been reported by Pelton and Chibante [1] and by Kawaguchi et al. [2] by investigating precipitation polymerization of N-isopropylacrylamide (NIPAM) as main monomer, methylenebisacrylamide (MBA) as a cross-linker and potassium persulfate (KPS) as the initiator. In addition, the effect of charged surfactant [sodium dodecyl sulfate (SDS)] on the precipitation polymerization has been examined and discussed by Pelton et al. [3]. The principal results have been discussed on the basis of the polymerization above the lower critical solution temperature (LCST) of the corresponding linear polymer, the uses of the cross-linker agent and charged initiator. Then, during the precipitation polymerization at a high temperature (above the LCST of the corresponding linear polymer), the precipitated macromolecule chains are cross-linked, leading to the formation of hydrogel particles. The main problem in the elaboration of thermally sensitive microgel is related to high production of water-soluble polymer [4, 5]. Various works have been also investigated to prepare core shell-like particles such as polystyrene core and poly(NIPAM) shell [6–9]. The effect of the used N-alkyalcrylamide or N-alkylmethacrylamide on surfactant-free radical emulsion polymerization of styrene has been examined, and the polymerization and the final colloidal properties reported have to be related to the amount of water-soluble monomer in the used recipe [9].
Regarding the colloidal and physico-chemical properties of such thermally sensitive particles, several authors have reported various works. To some extent, swelling ability [8], electrokinetic properties [10, 11], colloidal stability [12] have been systematically investigated as a function of temperature, pH of the medium, ionic strength and solvent nature. As expected, the reported results principally demonstrate the drastic effect of temperature on the investigated colloidal properties.
The great interest of functionalized thermally sensitive particles is based on the possible control of adsorption/desorption by motoring the incubation temperature, and consequently, the covalent binding efficiency of proteic materials [13]. Then, various functional thermally sensitive microgels have been reported. Whereas, few systematic studies have been dedicated to the elaboration of functionalized thermally sensitive latexes such as carboxylic [14], amine [15], boronic acid [15, 16]. Concerning the elaboration of thiol-containing thermally sensitive particles, the first preliminary work has been reported by Meunier et al. [17]. Colloidal particles bearing a thiol group is of great importance in biomedical diagnostic for specific and orientation of antibody fragments via disulfur bridging [18].
For a better understanding of the mechanistic approach of precipitation polymerization using NIPAM, MBA and protected thiol-containing monomer and colloidal properties of obtained latexes, a systematic polymerization study was of good interest.
The aim of the presented work is to investigate a systematic study to point out the role of each reactant and principally this related to the vinylbenzylisothiouronium chloride (VBIC) monomer on the precipitation polymerization process (of NIPAM, MBA, VBIC). In addition, the final latex particles such as particle size, size distribution, swelling ability and water-soluble polymer formation are addressed.
Experimental
Materials
N-isopropylacrylamide (NIPAM) from Kodak was recrystallized from toluene/hexane mixture. MBA from Amilabo was used without further purification. 2-2′-Azobis(2-amidinopropane) di-hydrochloride (V50) was provided kindly by Wako Chemical GmbH (Germany) and was recrystallized from water/acetone mixture. The VBIC monomer was synthesized according to Yamaguchi’s method [19] and was recrystallized from diethylether/ethanol mixture. Water is of Milli-Q grade (Millipore S. A., France) and was boiled for 2 h under a nitrogen stream before use. 14C-labeled iodoacetamide was from Amersham (France).

Preparation of latexes
The polymerizations were performed under batch condition and were carried out in a 250-ml thermostated reactor, round-bottomed four-necked flask, equipped with a glass anchor-shaped stirrer, condenser, thermocouple and nitrogen inlet. The stirrer, in all cases, was 300 rpm. Well-boiled deoxygenated water was introduced under a constant stream of nitrogen. The reaction temperature was controlled at 70 °C into the reactor. Water, NIPAM, MBA and VBIC were added and the temperature was checked to be constant at 70 °C; after equilibrium, V50, dissolved in water, was introduced. The overall conversions were determined gravimetrically. When opalescence appeared in the reactor, the stirrer was lowered to 100 rpm to avoid the flocculation of the latex particles. The duration of the polymerization was about 4 h.
Polymerization kinetics
NIPAM conversion was determined by Gas Chromatography (9000 Perkin). The column was a 10% carbowax, 20 M, onto chromosorb WAW 80 400, 2-m length. The detection was performed with flame ionization and the amount of NIPAM was referred to dimethylformamide used as external standard. The overall conversion and the water-soluble polymer (WSP) were determined gravimetrically.
Characterization of water-soluble polymer (WSP)
Average molecular weights, M w , were determined using statistical light scattering. In addition, 1H NMR technique (Brucker AC 200 spectrometer, 200 MHz) was used to analyze the microstructure and to quantify the amount of functional groups in the water-soluble polymer.
Volume phase transition temperature (T VPT) and lower critical solubility temperature (LCST) of microgels and water-soluble polymer were measured using UV-spectrophotometer Uvikon 941. The optical density (OD) was measured at a 540-nm wavelength as a function of temperature in a highly diluted concentration.
Colloidal characterization of latexes
Particle size was measured by quasi-elastic light scattering (QELS, using a N4MD from Coultronics). The temperature dependency of the hydrodynamic particle diameter was determined for all latexes by measuring the particle size as a function of temperature from 25 to 50 °C.
The VBIC concentration on the particles was examined using 14C-labeled iodoacetamide (from Amersham, France) [18] and Ellmann’s reagent [20]. 1H NMR technique was also used to examine the chemical composition of the water-soluble polymer.
Results and discussion
Batch polymerizations were investigated to point out the influence of each parameter on the polymerization kinetic, particle size, water-soluble polymer formation. Then, the influence of MBA, V50 and VBIC concentration were systematically studied.
Influence of cross-link concentration
At first, the influence of MBA concentration on the polymerization was investigated. The results of a series of latex preparation at 70 °C using a NIPAM concentration of 194.04 mmol/l, an initiator concentration of 1.2 mmol/l and an isothiouronium salt concentration of 0.1924 mmol/l are discussed below. The latexes prepared using high MBA concentration (36 mmol/l) show aggregation after 2-min polymerization only. The latexes prepared using MBA concentration below (36 mmol/l) remained stable during and after the polymerization reaction.
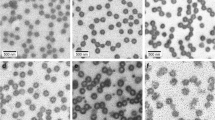
The diameter particle size was investigated by QELS (below and above the LCST of polyNIPAM) and by TEM analysis as a function of initial MBA concentration. The effect of MBA concentration revealed the existence of a maximum MBA limit that leads to unstable particles during the polymerization process as aforementioned. This phenomenon can be attributed to the high reactivity of such water-soluble cross-linker agent and to the possible bridging flocculation of particles via a chemical cross-linking process. As a general tendency, the hydrodynamic particle size increases with increasing amount of MBA as shown in Fig. 1. The swelling ability of the final particles decreases as the MBA amount increases in the polymerization recipe. This reflects the enhancement of the cross-linking density of the final particles vs MBA concentration.
The polymerization kinetic was studied as a function of initial MBA concentration by investigating the conversion vs time. The obtained results are reported in Fig. 2. The initial polymerization slop of polymer conversion vs time increases with increasing MBA concentration in the polymerization medium. The observed behavior is attributed to the reactivity of MBA, which acts as a water-soluble comonomer, whereas, the final conversion is found to reach a plateau value above 10 min polymerization time.
The influence of MBA amount on water-soluble polymer formation was also studied. As expected, the amount of water-soluble polymer decreases when increasing the initial MBA concentration in the polymerization recipe. This can be attributed to the effectiveness of MBA to cross-link the polymer chains (or nanoparticles) during the formation or nucleation step. Surprisingly, the amount of water-soluble polymer was found to be at less around 5 wt% in the investigated MBA range Fig. 3.
Influence of initiator concentration
The effect of the used water-soluble radical initiator (V50) on the final particle size was studied and the results obtained are reported in Fig. 4. In the investigated V50 concentration domain, the particle size (hydrodynamic diameters and TEM analysis) increases as the initiator amount increases in the polymerization medium. Compared to classical radical emulsion polymerization, the opposite effect was generally observed in low or moderate initiator concentration range. In such dispersion polymerization of water-soluble monomers, this can be attributed to the effect of salt induced by the initiator, which may affect the colloidal stability of the formed nanoparticles during the nucleation step, and leads to the enhancement of the final particle size.
The used radical initiator affects not only the final particle size, but also the swelling ability. In fact, the swelling capability increases as the amount of V50 initiator increases. This demonstrates the drastic influence of V50 on the microstructure of the microgels as also observed when the effect of MBA was investigated.
The influence of initiator concentration on the polymerization rate (data not shown) and on the water-soluble polymer formation (Fig. 5) was investigated. As expected, the polymerization rate (i.e., the initial slop of polymer conversion vs time) increases as the initiator amount increases in the polymerization recipe. With regard to the amount of water-soluble polymer formation, the increase of V50 amount leads to the enhancement of free-chain formation. This can be attributed to short chain formation, which are hard to precipitate on one hand and to incorporate on the formed particles, on the second hand. Consequently, in such precipitation polymerization, the increase of initiator concentration (in the presence of VBIC monomer) leads to a large particle size and to water-soluble polymer enhancement.
Influence of VBIC monomer concentration
The effect of VBIC comonomer concentration on the polymerization yield was examined and the results obtained are presented in Fig. 6 in which particles yield is reported as a function of polymerization time for various VBIC amounts ranging from 0 to 9.63 mmol/l.
The VBIC was found to have great influence on the polymerization kinetic (i.e. conversion vs time). For high VBIC amount, the crude final simples (i.e. prepared with 0.4815- or 2.4075-mmol VBIC) are transparent rather than turbid as for classical polymer latex particles. Rapid analysis by QELS reveals the absence of particles formation. 1H NMR and gas chromatography analysis pointed out that NIPAM monomer was totally consumed. Consequently, a high amount of VBIC monomer leads principally to the linear polymer rather than to the particles or nanogels and to a high polymerization conversion.
The analysis of water-soluble polymer by GPC analysis (after particles removal) revealed the formation of short polymer chains (i.e. short oligomers). The GPC traces were found to be well defined and symmetrical as for the monomer. The increase in VBIC concentration in the polymerization recipe leads drastically to a high water-soluble polymer formation (Fig. 7). Such behaviour has already been observed in the case of aminoethylmethacrylate hydrochloride (AEMH) comonomer used in a similar condition [5] and attributed to the transferring role of the protected amine compound. Then, the increase of water-soluble formation as a function of VBIC concentration can be attributed to the transferring character of this monomer even under the protected form.
The influence of VBIC monomer concentration of final particles size was also examined (Fig. 8). The particle size was found to be highly sensitive to VBIC amount in the recipe. A similar tendency has been observed when a water-soluble charged comonomer (such as amino-containing) was used in a similar batch precipitation polymerization [5]. This can be attributed principally to the influence of VBIC on the nucleation step by contributing to a high number of particle formation.
The swelling ability \({\left( {{\text{i}}{\text{.e}}{\text{.}}\,{\text{swelling}}\,{\text{ratio}} = {D_{{20\,{\text{ $^\circ $ C}}}} } \mathord{\left/ {\vphantom {{D_{{20\,{\text{ $^\circ $ C}}}} } {D_{{50\,{\text{ $^\circ $ C}}}} }}} \right. \kern-\nulldelimiterspace} {D_{{50\,{\text{ $^\circ $ C}}}} }} \right)}^{3}\) of the elaborated microgel particles decreases as the VBIC amount increases in the polymerization recipe. This can be attributed to a reduction of the final particle size and also to the enhancement of the cross-linking density of the formed particles. The rapid analysis of the molecular weight of the formed water-soluble polymer revealed interesting results. In fact, the molecular weight (M w ) decreases drastically from 67,800 g/mol for free VBIC to 31,600 g/mol for 0.0481-mmol VBIC, which confirms the formation of low polymer chains vs VBIC amount.
Conclusions
The preparation of thiol containing poly(N-isopropylacrylamide)-based particles was examined by investigating various parameters as a systematic study.
The polymerization kinetic was marginally affected by VBIC comonomer in the investigated concentration range. The tendency shows that the polymerization kinetic slightly increases with increasing VBIC amount in the polymerization recipe as evidenced by the initial slope of polymer conversion vs time. The VBIC monomer affects, principally, the particle yield and water-soluble formation. In fact, a low amount of VBIC monomer affects drastically the particle formation, and consequently, the final particle size. The water-soluble polymer was found to be related to various parameters. The increase of water-soluble polymer amount is associated to the use of a low concentration of the cross-linker agent, increasing the initiator amount and VBIC comonomer ratio over NIPAM.
It is interesting to notice that a weak amount of VBIC comonomer leads to a decrease in the average particle diameter, as generally observed in a radical surfactant-free emulsion polymerization when charged comonomer was used. The used monomer (VBIC) had a protected thiol function; then, we can assume that at the beginning of the polymerization reaction, the protected thiol was deprotected, and consequently, the resulting thiol acts as a good transfer agent. The formed oligomers during the first minutes of polymerization should have sufficient size to self-precipitate or to be cross-linked to form primary particles.
If this comonomer (VBIC) affects the polymerization, where is it located at the end of the polymerization? Three methods were used to detect the functional monomer on the particle surface. Even the isothiuronium salt was introduced in moderate concentration and whatever the explored analysis method, it was not detected on the particle surface. This phenomenon confirms that the salt was a transfer agent, and the monomer was mainly incorporated in the polymer chain and contributes to the polymer core formation and consequently, not accessible and detectable using classical techniques.
The volume phase transition temperature (T VPT) of the elaborated microgels was examined as a function of initial polymerization recipe. The T VPT temperature was not affected and found to be in the same range as for VBIC free microgels. Whereas, the LCST of the formed water-soluble polymer formed in the presence of VBIC comonomer was around 36 °C, slightly higher than that temperature that was generally observed in the case of linear homopoly(NIPAM) (32 °C).
References
Pelton RH, Chibante P (1986) Preparation of aqueous latices with N-isopropylacrylamide. Colloids Surf 20:247–256
Kawaguchi H et al (1988) Hydrogel microspheres I. Preparation of monodisperse hydrogel microspheres of submicron or micron size. Polym J 20:903–909
Mc Phee W, Tam KC, Pelton R (1993) (N-isopropylacrylamide) latices prepared with sodium dodecyl sulfate. J Colloid Interface Sci 156:24–30
Duracher D, Elaissari A, Pichot C (1999) Preparation of Poly(N-isopropylmethacrylamide) latexes kinetic studies and characterization. J Polym Sci Part A: Polym Chem 37:1823–1837
Meunier F, Elaissari A, Pichot C (1995) Preparation and characterization of cationic poly(n-isopropylacrylamide) copolymer latexes. Polym Adv Technol 6:489–496
Hoshino F et al (1987) N-substituted acrylamide–styrene copolymer latices. II. Polymerization behavior and thermosensitive stability of latices. Polym J 19(2):241–247
Senff H, Richtering W (1999) Rheology of a temperature sensitive core-shell latex. Langmuir 15:102–106
Kim JH, Ballauff M (1999) The volume transition in thermosensitive core-shell latex particles containing charged groups. Colloid Polym Sci 277:1210–1214
Duracher D et al (1998) Cationic amino-containing N-isopropylacrylamide–styrene copolymer latex particles. 1. Particle size and morphology vs polymerization process. Colloid Polym Sci 276:219–231
Pelton RH et al (1989) Particles size and electrophoretic mobilities of poly (N-isopropylacrylamide) latex. Langmuir 5:816–818
Nabzar L et al (1998) Electrokinetic properties and colloidal stability of cationic amino-containing N-isopropylacrylamide–styrene copolymer bearing different shell structures. Langmuir 14:5062–5069
Duracher D et al (1998) Cationic amino-containing N-isopropylacrylamide–styrene copolymer latex particles. 2. Characterization and colloidal stability. Colloid Polym Sci 276:920–929
Pichot C et al (2003) Functionalized thermally-sensitive polymer latex particles: useful tools for diagnostics. J Dispers Sci Technol 24(3–4):423–437
Duracher D et al (2000) Preparation of thermosensitive latexes by copolymerization of n-isopropylmethcrylamide with a chelating monomer. Macromol Symp 150:297–303
Elmas B et al (2004) Thermosensitive N-isopropylcarylamide–vinylphenyl boronic acid copolymer latex particles for nucleotide isolation. Colloids Surf A Physicochem Eng Asp 232:253–259
Hazot P et al (2002) Functionalization of poly(N-ethylmethacrylamide) thermosensitive particles by phenylboronic acid. Colloid Polym Sci 280:637–646
Meunier F, A Elaissari, Pichot C (2000) Synthesis of cationic and hydrophilic latexes: copolymerization of N-isopropylacrylamide and vinyl benzyl isothioronium hydrochloride salt. Macromol Symp 150:283–290
Delair T, Pichot C, Mandrand B (1994) Synthesis and characterization of cationic latex particles bearing sulfhydryl groups and their use in the immobilization of fab antibody fragments. Colloid Polym Sci 272:72–81
Yamaguchi K et al (1987) Makromol Chem Rapid Commun 8(230)
Ellmann GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Meunier, F., Pichot, C. & Elaïssari, A. Effect of thiol-containing monomer on the preparation of temperature-sensitive hydrogel microspheres. Colloid Polym Sci 284, 1287–1292 (2006). https://doi.org/10.1007/s00396-006-1514-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-006-1514-7