Abstract
Water-soluble, amphiphilic diblock copolymers were synthesized by reversible addition fragmentation chain transfer polymerization. They consist of poly(butyl acrylate) as hydrophobic block with a low glass transition temperature and three different nonionic water-soluble blocks, namely, the classical hydrophilic block poly(dimethylacrylamide), the strongly hydrophilic poly(acryloyloxyethyl methylsulfoxide), and the thermally sensitive poly(N-acryloylpyrrolidine). Aqueous micellar solutions of the block copolymers were prepared and characterized by static and dynamic light scattering analysis (DLS and SLS). No critical micelle concentration could be detected. The micellization was thermodynamically favored, although kinetically slow, exhibiting a marked dependence on the preparation conditions. The polymers formed micelles with a hydrodynamic diameter from 20 to 100 nm, which were stable upon dilution. The micellar size was correlated with the composition of the block copolymers and their overall molar mass. The micelles formed with the two most hydrophilic blocks were particularly stable upon temperature cycles, whereas the thermally sensitive poly(N-acryloylpyrrolidine) block showed a temperature-induced precipitation. According to combined SLS and DLS analysis, the micelles exhibited an elongated shape such as rods or worms. It should be noted that the block copolymers with the most hydrophilic poly(sulfoxide) block formed inverse micelles in certain organic solvents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amphiphilic block copolymers suited for self-organization in aqueous media are composed of hydrophobic and hydrophilic blocks. Within the class, the most widely studied samples are amphiphilic diblock copolymers, i.e., such with one water-soluble and one water-insoluble block. Their reversible aggregation process in water is analogous to the micellization of low-molar-mass surfactants and is generally assumed to occur via a so-called closed association process [1]. A typical feature, which makes amphiphilic block copolymers particularly attractive in comparison to their low-molar-mass counterparts are their much lower critical micelle concentrations (CMCs), rendering such polymeric micelles very stable upon dilution [1]. In the simplest case, their self-assembly produces spherical micelles consisting of the core made of the hydrophobic block and a flexible corona made of the hydrophilic block (see Fig. 1a). Dependent on the relative lengths of the blocks, two extreme cases are distinguished: the so-called “crew-cut” micelles and the “hairy” micelles. For highly voluminous hydrophobic domains, bilayer morphologies such as vesicles can be formed (Fig. 1b).
Amphiphilic diblock copolymers were reported to form aggregates in aqueous media with more than 30 different morphologies [2]. Numerous reports have established relations between the morphology of the aggregates formed and structural parameters such as the polarity of each block [1], the rigidity of the blocks [3], the relative lengths of the blocks [4–8], the overall molar mass of the polymers [9], or the polydispersity of the molar mass distribution of the blocks [10, 11]. Moreover, the preparation conditions such as the initial polymer concentration [12, 13] or the cosolvent used in the dialysis technique [12, 14] were shown to be crucial for the micellar characteristics. Moreover, the aggregation behavior depends on external factors, such as temperature [12, 15] or the ionic strength of the medium [16].
In the past, amphiphilic block copolymers used to be synthesized preferentially by the methods of living anionic and cationic polymerization. This restricted the chemical nature of the hydrophilic blocks to few systems. However, the recent techniques of controlled free-radical polymerization (such as reversible addition fragmentation chain transfer “RAFT”) have considerably simplified the synthesis of well-defined macrosurfactants, thus enormously increasing the chemical diversity, in particular, of the hydrophilic block [17].
This work deals with the self-assembly properties of three types of nonionic amphiphilic diblock copolymers polymerized via the RAFT method, in water, and in selective organic solvents. The diblock copolymers are composed of poly(butyl acrylate) [poly(M1)] as constant hydrophobic block and of three different nonionic hydrophilic blocks, namely, thermally sensitive poly(N-acryloylpyrrolidine) [poly(M2)], poly(dimethylacrylamide) [poly(M3)], and strongly hydrophilic poly(acryloyloxyethyl methylsulfoxide) [poly(M4)] (see Table 1). The aggregation properties of the amphiphilic diblock copolymers in water and in organic solvents were studied as a function of the macromolecular parameters varying between the different systems, i.e., the nature of the hydrophilic block and the relative and absolute molar masses of the blocks. The influence of experimental factors such as preparation conditions and temperature on the micellar characteristics was studied, too, and the stability of the micelles upon dilution and against aging was investigated.
Experimental section
Materials and methods
The synthesis and molecular characterization of the monomers M2 [18] and M3 [19] and the block copolymers [18, 20] are reported elsewhere. The composition of the diblock copolymers was determined by the ratio X of the integrals of NMR peaks of protons of each block. The results were confirmed by elemental analysis [20]. The overall mass of the block copolymers was then calculated from the ratio X and the molar mass of poly(M1), previously determined by size exclusion chromatography in tetrahydrofuran (THF) [20]. The molar mass distribution polydispersity indexes (PDI) of the block copolymers were determined by size exclusion chromatography in N-methyl pyrrolidone (see Table 1).
Micellar solutions were prepared using water purified by a Millipore Qplus water purification system (resistance 18 MΩ cm) via the dialysis method. The polymers were first dissolved in a cosolvent for 24 h (see Table 2) and then dialyzed against purified water for 3 days (membranes “Zellu Trans” with nominal molar mass cut off of 3,500). The concentration of the solutions was determined by gravimetry after lyophilization of 20 ml and adjusted to 1 g·l−1. For the preparation of inverse micelles, the polymers were directly dissolved in the selective organic solvent with a concentration of 1 g·l−1.
1H (300 MHz) NMR spectra were taken with an apparatus Bruker Avance 300 (128 scans for 1H). Differential scanning calorimetry (DSC) was performed with a DSC 822 (DSC, Mettler Toledo, USA) under nitrogen atmosphere using a heating rate of 20 °C·min−1. The glass transition temperature (T g) was evaluated as the midpoint temperature of the characteristic heat capacity change seen during the second heating run.
Dynamic light scattering (DLS) was performed with a high performance particle sizer (Malvern Instruments) equipped with an He–Ne laser (633 nm) and a thermoelectric Peltier temperature controller (temperature control range 10–90 °C). Before measurement, the polymer solutions were filtered using a Sartorius Ministar-plus 0.45 μm disposable filter and were placed in a polystyrene (water) or glass cuvette (organic solvent). Measurements were made at the scattering angle θ=173° (“backscattering detection”) at 25 °C. The autocorrelation functions were analyzed with the CONTIN method. The apparent hydrodynamic diameters were calculated according to the Stokes–Einstein equation:
where D app is the apparent diffusion coefficient and η is viscosity of the solution.
Static light scattering (SLS) for the characterization of micellar solutions was performed with a Sofica instrument equipped with a He–Ne laser (λ=633 nm). The scattered light intensity was recorded at scattering angles from 30 to 145° at 5° intervals. All measurements were performed at 25 °C (±0.1 °C). Water was used for calibration to determine the scattering volume corrected Rayleigh ratio. Ten milliliters of aqueous micellar solution was filtered with a Sartorius Ministar-plus 0.45 μm disposable filter and subsequently placed into a cylindrical quartz cuvette. The cuvettes were extensively cleaned with acetone and distilled water to completely remove any traces of block copolymers. For the analysis of the SLS data, the Zimm equation was used [21]:
where K is an optic constant depending on the refraction index increment of the solution, c is the concentration of the solution, R(θ) is the Rayleigh ratio (the angle between incident beam and scattered beam is 2θ), \(M^{m}_{w}\) is the weight-average molar mass of the micelles, q is the norm of the scattering vector, and A 2 is the second virial coefficient of the osmotic pressure.
Results and discussion
Structure of the amphiphilic block copolymers
To facilitate meaningful studies of their aqueous self-assembly, the amphiphilic block copolymers were designed such that the hydrophobic block has a glass transition temperature below 0 °C. The hydrophilic block was designed to be nonionic, avoiding groups with acidic hydrogens, which can undergo self-consistent hydrogen bonding. Accordingly, three nonionic hydrophilic blocks of different polarity and increasing hydrophilicity were chosen, namely, poly(N-acryloyl pyrrolidine) poly(M2), poly(dimethyl acrylamide) poly(M3), and poly(2-(acryloyloxy ethyl) methyl sulfoxide) poly(M4) (Table 1).
Within this group, poly(dimethyl acrylamide) poly(M3) is a well established hydrophilic block [18, 22, 23]. The polyacrylate poly(M4) was hardly used so far as hydrophilic block although it is even much more hydrophilic than polyacrylamide poly(M3) by virtue of the strongly hydrophilic sulfoxide moiety [19, 24]. As poly(M4) exhibits low toxicity [25, 26], this polymer is in fact an attractive candidate for the incorporation in amphiphilic diblock copolymers, for instance, with possible perspectives for cosmetic or biomedical applications. The seldom used substituted polyacrylamide poly(M2) is the least hydrophilic block investigated, exhibiting a lower critical solution temperature (LCST) of about 57 °C in water at ambient pressure [18, 27, 28]. Poly(butyl acrylate) poly(M1) was selected as hydrophobic block throughout this study as it exhibits a low glass transition temperature T g of about −55 °C [1]. This may eventually confer a dynamic character to aqueous micellar solutions.
In our study, the lengths of both the hydrophobic and hydrophilic blocks were varied systematically. As the aggregation of block copolymers is typically more sensitive to small changes in the hydrophobic block than in the hydrophilic block [1], identically long hydrophobic blocks are advantageous for comparative studies. This implies that the hydrophobic blocks should be synthesized first and then used as macro-RAFT agents in the second step in which the hydrophilic blocks are added and varied [20]. As acrylic monomers were chosen for all blocks, the detailed sequence of the monomer addition should not be problematic [29]. But inherently, the RAFT technique leads to the incorporation of the fragments of the RAFT agent into the macromolecules, determining virtually all end groups of the block copolymers. Therefore, the reinitiating group “R” of a dithioester Z-CSS-R used as RAFT agent will end up as end group attached to the hydrophobic block, while the Z-CSS-group will always be attached to the end of the hydrophilic block. Accordingly, the R-group should be reasonably hydrophobic whereas the Z-CSS-group should be at least moderately polar, apart from satisfying the general reactivity rules of the “R” and “Z” groups to guarantee successful controlled free radical polymerizations [30–34]. Within this reasoning, we decided for the RAFT agent benzyl dithiophenylacetate BDTPhA [18, 20], which improves not only the polymerization kinetics compared to the mostly used dithiobenzoates [35–39], but which also provides a hydrophobic benzyl moiety as end group of the hydrophobic block, and the moderately polar dithiophenylacetate moiety as end group of the hydrophilic block (cf. Table 1). Accordingly, the polymer end groups should not interfere with the inherent tendency of the hydrophilic and hydrophobic blocks to self-organize in aqueous media.
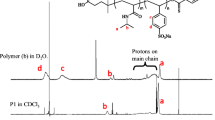
The copolymers were successfully synthesized and isolated in pure form [20]. They are all soluble in CHCl3, which is a good solvent for all blocks studied here. The different molecular fragments are clearly discernable in the H-NMR spectra in CDCl3 (Fig. 2). Table 1 lists the various amphiphilic diblock copolymers studied in this work. We define the composition parameter f as the ratio of the degrees of polymerization of the hydrophilic and the hydrophilic blocks \({\left( {f = {N_{{hydrophilic}} } \mathord{\left/ {\vphantom {{N_{{hydrophilic}} } {N_{{hydrophobic}} }}} \right. \kern-\nulldelimiterspace} {N_{{hydrophobic}} }} \right)}.\)
Compatibility studies of the block copolymers in bulk
Before studying the self-organization in aqueous media, we considered it important to know about the compatibility of the polymer blocks constituting the polymeric surfactants with each other. The compatibility of the two blocks, expressed by the factor χN (with χ as the Flory–Huggins interaction parameter and N as the overall degree of polymerization), governs their aggregation properties not only in bulk but also in a selective solvent [1]. Conveniently, the compatibility of the individual blocks in block copolymers can be studied via their thermal properties [12, 40–43], as for instance, determined by DSC. For all block copolymers studied, namely, poly(M1-b-M2), poly(M1-b-M3), and poly(M1-b-M4), two distinct T gs are observed. The first transition, in the temperature range from −49 to −46 °C, is attributed to the T g of the hydrophobic block poly(M1) that exhibits a T g at −47 °C in its pure form [20]. The second glass transition at much higher temperatures corresponds to the T g of the hydrophilic block employed, namely, T g=142 °C for poly(M2), T g=105 °C for poly(M3), and T g=25–30 °C for poly(M4). This thermal behavior is exemplified in Fig. 3, showing the DSC traces of (M1) 95 -b-(M2) 157 (a) and (M1) 86 -b-(M3) 138 (b).
The occurrence of the two T gs in the three diblock copolymer systems studied demonstrates that the hydrophilic and hydrophobic blocks selected are immiscible and form already different microdomains in bulk. This is supposed to favor microphase separation in solution and consequently, the aggregation of the block copolymers into micelles in a selective solvent.
Self-assembly behavior of the diblock copolymers
Aggregation in water
General features Typically, the self-assembly properties of amphiphilic block copolymers in selective solvents such as water depend on their composition and the protocol used for the preparation of the micellar solutions [1]. Thus, the effect of these two factors was studied for the various block copolymers. The dialysis technique was preferred for the preparation of aqueous micellar solutions because it allows the continuous and slow exchange of solvents, avoiding—or at least minimizing—the formation of large aggregates [1]. The influence of the common solvent used to dissolve the block copolymers before dialysis was studied, comparing dioxane (Table 2A), THF (Table 2B), and dimethylacetamide (Table 2C).
Chloroform was studied for the dialysis technique, too, as it is also a good solvent for all blocks under investigation (cf. Fig. 2). But it is worth noting that several systems precipitated during the dialysis process, like (M1) 86 -b-(M3) 138 , (M1) 133 b-(M3) 146 , and (M1) 133 -b-(M4) 106 , if chloroform was used to dissolve the copolymer before dialysis. This may be explained by the fact that chloroform is not fully miscible with water, in contrast to the other solvents investigated. Many systems turned slightly cloudy soon after preparation, implying the formation of large aggregates. As a general tendency, this was the case for polymers with longer hydrophobic blocks than the hydrophilic ones (f<1) or when the cosolvent used before dialysis was not a good solvent for one of the block.
The influence of the copolymer composition and the preparation conditions was investigated in more details using DLS. The samples were followed over long time periods. As shown in Table 2, the various amphiphilic block copolymers form aggregates in the nanometer range with hydrodynamic diameters between 20 and 130 nm. The micelle-like aggregates formed were stable over long time periods as demonstrated by the DLS analysis at 7 months after preparation. Cloudy solutions exhibited bimodal or trimodal particle size distributions, i.e., notable amounts of large aggregates (>200 nm) coexisting with small micelles. Although this kind of aggregates is a well-known problem encountered in the self-assembly of block copolymers, their origin is not yet clear [8, 44]. These large aggregates seem to disaggregate with time and almost disappeared after 3 or 7 months of storage at ambient temperature with no change of the micelle size (Table 2). The aqueous solution of block copolymer (M1) 95 -b-(M2) 157 prepared by dialysis of a polymer solution in THF against water exemplifies this process (see Table 2B). The large aggregates (D H=295 nm) present directly after preparation disappeared progressively with time. After 7 months, only micelles (D H=40 nm) were observed. The observations imply that the systems need extended times for equilibration. This can be attributed to the much slower diffusion and exchange rates of amphiphilic block copolymers compared to molar mass surfactants [1]. Furthermore, the disappearance of the large aggregates with time, in favor of micelles, suggests that the micellization of the block copolymers is thermodynamically favored.
Note that the micellization becomes thermodynamically unfavorable for particularly low values of f if the hydrophilic block is not made of very hydrophilic monomers. For example, (M1) 86 -b-(M3) 125 (f=1.45) formed micelles (D H=60 nm) and bigger aggregates directly after dialysis and only micelles (D H=54 nm) at 3 months after preparation. But an aqueous solution of (M1) 133 -b-(M3) 146 (f=1.09), though prepared according to the same protocol, precipitated within 3 months after preparation. Hence, there seems to be a critical value of f for a given hydrophilic block, below which the formation of large precipitating aggregates is favored, while for higher values, micellization is thermodynamically favored. In contrast, a solution of (M1) 133 -b-(M4) 53 (f=0.40) with a much lower f value only contained micelles (D H=93 nm) after 3 months equilibration time but did not precipitate. This different behavior can be explained by the much higher hydrophilicity of the sulfoxide groups in poly(M4) compared to the tertiary amide groups in poly(M3) [19].
The effect of the solvent used before dialysis on the final state of the polymer aqueous solutions differed between the samples as a function of their macromolecular composition. For the weakly hydrophilic block poly(M2), no difference was observed between dioxane and THF after equilibration, although THF seemed more suitable to minimize the initial formation of large aggregates. For the more hydrophilic block poly(M3), the use of dioxane as cosolvent before dialysis was the most suited protocol to obtain monomodal micellar systems after equilibration. In the case of higher molar mass poly(M1) blocks, i.e., for (M1) 133 , the use of THF instead of dioxane did not allow to avoid the inevitable precipitation of the sample. The micellization of block copolymers composed of the most hydrophilic block of this study, poly(M4), was favored when dimethylacetamide was used as cosolvent before dialysis. As shown in Table 2A, dioxane led only to mixtures of micelles and large aggregates, suggesting that it is not a good solvent for the hydrophilic block. These observations underline the importance of the preparation conditions for the aggregation of amphiphilic block copolymers in water, as often described [41, 45–48].
Such a strong dependence of the micellar characteristics on the preparation conditions of the aqueous polymer solutions is typical for block copolymers, particularly when frozen micellar systems are formed, i.e., without unimer exchange between the micelles [1]. Typically, such systems are formed if the core-forming blocks exhibit a relatively high glass transition temperature (T g) [1, 49]. A priori, this is not the case here because poly(M1) exhibits a T g of about −50 °C. Nevertheless, the low T g of the core-forming block seems to be a necessary but not sufficient condition for micellar exchange. For instance, Winnik and coworkers reported the presence of frozen micellar aggregates although there is low T g on the core chains [50]. This was attributed to the strong segregation of the polymer segments in the selective solvent used, associated with high values of χN [16]. In this scenario, micellar exchange can be suppressed. As shown elsewhere, the micellar systems studied exhibit a strong segregated thermodynamic state due to the high incompatibility of the blocks constituting the macrosurfactants [20].
The investigation of the micellar exchange dynamics of the systems studied, via the formation of “mixed” micelles, is reported elsewhere [20]. Briefly, two micellar solutions of two different polymers or of one polymer and one standard low-molar-mass surfactant with different D H were mixed and stirred for 3 days at 25 °C and characterized by DLS. Micelles with monomodal size distribution were formed after mixing with D H values comparable to the D H values of the initial larger micelles. This indicated the formation of mixed micelles and shows that unimer exchange occurred between the two populations of micelles. This proved the mobile character of the micellar systems studied, provided by the mobility of the chains of the low T g hydrophobic block poly(M1) at 25 °C, although in a highly segregated state.
Influence of environmental parameters The effect of concentration and of temperature on the self-assembly of the diblock copolymers in water was studied by DLS, too. The aqueous micellar solutions of diblock copolymers were diluted 105 times (i.e., to a concentration of 10−5 g·l−1) and characterized by DLS after 3 days and after 3 months. As depicted in Fig. 4, no change of the aggregate size was observed.
Because the systems are not frozen as previously evoked, the good stability of the micelles upon extreme dilution suggests that the block copolymers studied exhibit a very low CMC or do not exhibit any CMC. In any case, this confirms that micelles of macrosurfactants do not suffer from the problematic dilution effects encountered with low-molar-mass surfactants [1]. This is a great advantage for applications such as controlled drug delivery systems, for instance, where the micellar drug carrier is, e.g., confronted to high dilution effects in the bloodstream [51].
As described elsewhere [18, 20], the behavior of the polymeric micellar systems studied toward temperature depended on the nature of the hydrophilic block. Hydrophilic blocks such as poly(M3) or poly(M4), which do not exhibit any LCST under 100 °C, confer the micellar systems a particularly good stability upon temperature cycles. A slight decrease of D H of about 5 nm with increasing temperature from 25 to 80 °C was observed due to a lower solvation of nonionic hydrophilic blocks in the corona of the micelles at high temperature [20]. This process was reversible. In the case of the block copolymer poly(M1)-b-poly(M2), increasing temperature to 51 °C resulted in a gradually reduced hydrodynamic diameter, too. But further heating above 51 °C caused an irreversible precipitation of the sample due to the LCST of poly(M2) at 57 °C [18]. Note that the cloud point of the block copolymer at the same concentration of 0.1% was lower than the LCST of homopolymer poly(M2). Such an interdependence of the thermal behavior of thermoresponsive blocks on the other molecular fragments in polymeric amphiphiles should be kept in mind when designing new thermoresponsive macrosurfactants.
Correlations between the micellar size and the copolymer composition The micellar D H values determined by DLS experiments were first correlated with the absolute lengths of the hydrophobic block. Note that the micellar aggregates formed might exhibit other geometries than spheres. Consequently, the D H values determined by DLS are only apparent values. As depicted in Fig. 5, the apparent micelle size in water increases with increasing length of the hydrophobic block and, this being independent of the nature of the hydrophilic block, is in agreement with many reports [4, 6, 8, 52].
The influence of the length of the corona-forming block on the micellar size is more difficult to elucidate. It seems to depend on the nature of the hydrophilic block (see Table 2). The results are conclusive only for the block copolymers poly(M1)-b-poly(M3). For a given length of the hydrophobic block, D H increases with increasing length of the block poly(M3). This can be exemplified by the comparison of the block copolymers (M1) 37 -b-(M3) 70 (D H=21 nm) and (M1) 37 -b-(M3) 145 (D H=36 nm). The finding can be explained by a more stretched conformation of the longer hydrophilic chains, thus increasing the micellar size [53]. Unfortunately, the results are not conclusive for the series of copolymers bearing the strongly hydrophilic block poly(M4) as increasing molar masses of the block copolymers lead to large aggregates, which do not exhibit a spherical shape (see below). In any case, the absolute length of the hydrophobic block poly(M1) is the main factor governing the apparent micellar size, much more than the size of the hydrophilic block.
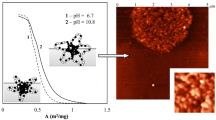
Study of the micellar shape As previously evoked, DLS measurements provide only apparent values of D H and do not give information about the true shape of the micelles. For this reason, the micellar solutions were characterized by SLS, too. The form of the so-called Zimm plot [21] and the ratio R g/R h determine the morphology of the polymeric aggregates. Values of the ratio R g/R h inferior to 1 are typical for spheres (theoretical value for compact spheres 0.775 and <0.775 soft-spherical structures), values equal to 1 indicate vesicles, and values superior to 1 are characteristic for “elongated” micelles, i.e., prolate ellipsoids, worm-like or rod-like micelles [14, 45, 49, 54–58]. R g values were calculated from the slope (R g 2/3) of the Zimm plots. The R g and R g/R h values obtained are summarized in Table 3.
The Zimm plots of all the samples except (M1) 133 -b-(M4) 53 exhibited the same negative deviation from linearity as exemplified by the Zimm plot depicted on Fig. 6a for (M1) 85 -b-(M3) 125 . This curve form is typical for rods. This was confirmed by R g/R h values clearly above 1, typical for rods, too [49, 55].
The very high R g/R h values are surprising at a first view. These values may be explained by the polydispersity of the micellar aggregation numbers, which tends to shift the aggregate size values determined by light scattering to higher values in a more pronounced way for R g than for R h, [45]. In any case, whatever the nature of the hydrophilic block, the micelles exhibit a rod-like shape. In the light of the relatively high f values of the polymers studied by SLS (cf. Table 1), the repulsive interactions between the solvated polar chains seem to be limited so that the assembly is elongated in one dimension [49]. Indeed, it is known that the stretching of the corona chains results in a destabilization of the spherical shape, giving rise to rod-like morphologies [55]. Note that the copolymers with hydrophilic blocks of poly(M4) have smaller R g/R h values than such with hydrophilic blocks of poly(M2) and poly(M3). This can be attributed to high hydrophilicity of the sulfoxide moieties in poly(M4), which results in a better solvation of the corona chains, thus favoring aggregates with a prolate shape, i.e., with a “more spherical” shape.
Block copolymer (M1) 95 -b-(M4) 190 behaved exceptionally, exhibiting a Zimm curve whose form differs from one of the systems described above (see Fig. 6b). Furthermore, a R g/R h value of 1.1 was measured, which might indicate the presence of hollow spheres such as vesicles, or of soft spheres. At present, we cannot distinguish between these two possibilities. The formation of vesicles is generally correlated with voluminous hydrophobic blocks attached to small hydrophilic ones [10]. Therefore, aggregation to vesicles by (M1) 95 -b-(M4) 190 seems less probable as this copolymer is characterized by a high f value of 2.
Self-assembly properties in organic solvents
In comparison to the micellization behavior of amphiphilic block copolymers in aqueous medium, little attention was paid to their self-assembly in nonaqueous solvents. Two cases have to be distinguished: (1) micellization in nonaqueous highly polar solvents (e.g., short chain alcohols and formamide) where direct micelles with the polar block as corona are formed [59, 60] and (2) micellization in solvents with moderate to low polarity (e.g., butyl acetate and toluene), which are selective for the hydrophobic block, and where inverse micelles with the apolar block as corona are formed [55].
1H-NMR spectroscopy enables a fast, preliminarily test to verify the aggregation of a block copolymer in selective solvents [53, 61, 62]. Figure 7 exemplifies this method for block copolymer (M1) 133 -b-(M4) 53 . The NMR-spectra of (M1) 133 -b-(M4) 53 were compared in chloroform (cf. Fig. 2a), a good solvent for both blocks; in DMSO (Fig. 7a), a priori a selective solvent for poly(M4); and in acetone (Fig. 7b), a priori a selective solvent for poly(M1).
The spectra clearly illustrate that the signals of the protons of the hydrophobic block (e.g., protons “1” or “4”), which are well resolved in chloroform, are broadened and apparently loose intensity in DMSO (Fig. 7a). This suggests that these protons are located in a poorly solvated microenvironment, i.e., in the core of aggregates, indicating the formation of aggregates in DMSO. The comparison of Figs. 2a and 7b demonstrates the opposite effect in acetone. The signals of protons 7 and 8 of the hydrophilic block of polymer (M1) 133 -b-(M4) 93 are well visible and resolved in chloroform, whereas they virtually disappear in acetone. This confirms that acetone is a nonsolvent for poly(M4) as observed via solubility tests with a homopolymer poly(M4). Moreover, the NMR-spectra indicate that (M1) 133 -b-(M4) 93 aggregates into inverse micelles in acetone with a core of poly(M4) and a corona of poly(M1). Note that self-assembly in acetone is exceptional for amphiphilic block copolymers containing poly(M4). The other block copolymers studied, bearing polyacrylamides as hydrophilic block, did not produce micelles in acetone according to 1H-NMR spectroscopy measurements. The different behavior of the new hydrophilic block poly(M4) may be due to the high dipole moment of the sulfoxide moiety [19].
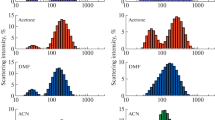
The aggregation of the diblock copolymers in organic solvents was confirmed by DLS studies. The macrosurfactants were directly dissolved in three solvents of different polarities, namely, DMSO, acetone, and THF. The DLS data of polymer solutions are summarized in Table 4.
The results demonstrate that the associative behavior of the diblock copolymers strongly depends on their composition and on the nature of the solvent used. The presence of small amounts of large aggregates is attributed to the preparation method of the solutions not using the dialysis technique. Block copolymers (M1) 95 -b-(M2) 157 and (M1) 86 -b-(M3) 138 behaved similarly in the different organic solvents. This can be explained by the relatively close polarity of their hydrophilic blocks and of their f values. In DMSO, they formed presumably micellar aggregates with a D H of 31 and 38 nm, respectively. In agreement with the NMR spectra taken in this solvent, this means that poly(M1) is not dissolved in DMSO, whereas poly(M2) and poly(M3) exhibited a good solubility in DMSO. Thus, one can conclude that block copolymers poly(M1)-b-poly(M2/M3) form direct micelles in DMSO. In contrast, the polar blocks poly(M2) and poly(M3) are well dissolved in acetone and THF as indicated by D H values between 6 and 9 nm, typical for unimolecular polymer coils. This finding is surprising as the hydrophobic blocks poly(M1) are soluble in these solvents [20], but homopolymers poly(M3) precipitate from THF and acetone. Thus, the formation of inverse micelles could be expected in these solvents. But despite relatively high f values, the solvophobic interactions between the chains of these blocks are apparently too low in comparison to the stretching of the poly(M1) chains in THF or acetone to form micellar aggregates.
As indicated by the 1H-NMR measurements, the situation for block copolymers poly(M1)-b-poly(M4) is markedly different. In DMSO, (M1) 133 -b-(M4) 93 formed micelles with a D H of 50 nm, whereas (M1) 37 -b-(M4) 106 was well-solubilized. This behavior can be directly correlated to the composition of the polymers. With a f value of 0.7, the solvophobic interactions of (M1) 133 dominate the behavior of the large block copolymer in solution, leading to micellization. In contrast with a f value of 2.9, the affinity of (M4) 106 to DMSO is dominating, preventing the short solvophobic block from aggregation in the selective solvent. This means that a critical value of f exists for block copolymers poly(M1)-b-poly(M4), above which the block copolymer is apparently well solubilized and below which micellization occurs in DMSO. In acetone, the DLS data indicated the presence of inverse micelles with a D H of 50 nm for (M1) 133 -b-(M4) 93 and of 94 nm for (M1) 37 -b-(M4) 106 as corroborated by NMR experiments. Note that a D H value of 94 nm for (M1) 37 -b-(M4) 106 is larger than the theoretical sphere with fully stretched chains (D th=72 nm), suggesting the formation of nonspherical micelles or larger aggregates. This can be explained by the much higher ratio of solvophobic block to solvophilic block than in the case of (M1) 133 -b-(M4) 93 , leading to an increase of the packing parameter, which would destabilize the spherical shape. The behavior of poly(M1)-b-poly(M4) in THF seems to depend on the copolymer composition, too. For high values of f, i.e., for short poly(M1) blocks, precipitation occurs. For values of f smaller than 1, inverse aggregates were formed: (M1) 133 -b-(M4) 93 aggregates into inverse micelles with a D H of 67 nm. This behavior is consistent with the observations made in the opposite situation, i.e., in water. Too large solvophobic domains can lead to macroscopic phase separation. Furthermore, the larger aggregate size in THF than in acetone for this sample may be explained by the increasing solvent quality for the core-forming block, resulting in partial swelling.
Conclusions
The self-organization of three series of amphiphilic diblock copolymers sharing poly(butyl acrylate) as hydrophobic block with three different nonionic hydrophilic blocks were studied in bulk and in solution. In bulk, the occurrence of two glass transition temperatures indicated the immiscibility and thus the high incompatibility of the blocks. The characterization of the micelle-like aggregates formed in water as a function of time confirmed the thermodynamically favored microphase separation process. Though the highly segregated thermodynamic state, the micellar system is a priori dynamic due to the low glass transition temperature of the hydrophobic block. Nevertheless, the experimental preparation conditions strongly influenced the self-assembly of the diblock copolymers in water and the micellar characteristics. Supported by the high stability of the micelles upon dilution on the one hand, and the dynamic character of the micellar systems on the other hand, the macrosurfactants studied exhibit very low CMCs below the detection limit. Hydrophilic blocks poly(dimethyl acrylamide) and a new sulfoxide polymer conferred the micellar systems a high stability upon temperature cycles, in contrast to macrophase separation observed with poly(N-acryloyl pyrrolidine) block, which exhibits a LCST in water. Correlations between the micellar size and the block copolymer compositions showed that the absolute length of the hydrophobic block is the main factor governing the micellar size. Nevertheless, a minimum hydrophilic block is needed to avoid precipitation of the aggregates upon storage. This minimum size is smaller for more hydrophilic blocks. In the most cases, the micelles exhibited a rod-like morphology. Finally, all copolymers with sufficiently long solvophobic blocks aggregated into direct micelles in DMSO. In addition, the high polarity of the sulfoxide block resulted in the formation of inverse micelles in acetone and THF, too.
Abbreviations
- D H :
-
hydrodynamic diameter of micelles
- DLS:
-
dynamic light scattering
- DMSO:
-
dimethylsulfoxide
- LCST:
-
lower critical solution temperature
- NMR:
-
nuclear magnetic resonance
- RAFT:
-
reversible addition fragmentation chain transfer
- R g :
-
gyration radius of micelles
- R h :
-
hydrodynamic radius of micelles
- SLS:
-
static light scattering
- T g :
-
glass transition temperature
- THF:
-
tetrahydrofuran
References
Riess G (2003) Prog Polym Sci 28:1107
Cameron NS, Corbierre MK, Eisenberg A (1999) Can J Chem 77:1311
Loos K, Böker A, Zettl H, Zhang M, Krausch G, Müller AHE (2005) Macromolecules 38:873
Förster S, Zisenis M, Wenz E, Antonietti MJ (1996) Chem Phys 104:9956
Zheng Y, Won YY, Bates FS, Davis HT, Scriven LE, Talmon YJ (1999) Phys Chem B 103:10331
Jain S, Bates FS (2004) Macromolecules 37:1511
Lim Soo P, Eisenberg AJ (2004) J Polym Sci Part B Polym Phys 42:923
Stenzel MH, Barner-Kowollik C, Davis P, Dalton HM (2004) Macromol Biosci 4:445
Kaya H, Willmer L, Allgaier J, Stellbrink J, Richter D (2002) Appl Phys A Mater Sci Process 74:499
Terreau O, Bartels C, Eisenberg A (2004) Langmuir 20:637
Jiang Y, Chen T, Ye F, Liang H, Shi AC (2005) Macromolecules 38:6710
Yang Z, Yuan J, Cheng S (2005) Eur Polym J 41:267
Förster S, Antonietti M (1998) Adv Mater 10:195
Zhang W, Shi L, An Y, Gao L, Wu K, Ma R (2004) Macromolecules 37:2551
Won YY, Davis HT, Bates FS (2003) Macromolecules 36:953
Förster S (1997) Ber Bunsenges Phys Chem 101:1671
Matyjaszewski K, Davis TP (eds) (2002) In: Handbook of radical polymerization. Wiley-Interscience, Hoboken
Mertoglu M, Garnier S, Laschewsky A, Skrabania K, Storsberg J (2005) Polymer 46:7726
Hennaux P, Laschewsky A (2003) Colloid Polym Sci 281:807
Garnier S, Laschewsky A (2005) Macromolecules 38:7580
Zimm BHJ (1948) Chem Phys 16:1093
Fischer A, Brembilla A, Lochon P (2001) Polymer 42:1441
Nowakowska M, Szczubialka K, Grebosz M (2003) J Colloid Interface Sci 265:214
Hennaux P, Laschewsky A (2001) Colloid Polym Sci 279:1149
Hofmann V, Ringsdorf H, Muacevic G (1975) Makromol Chem 176:1929
Hofmann V, Ringsdorf H (1980) Makromol Chem 181:351
Storsberg J, Laschewsky A (2004) SÖFW-J 130:14
Ito S, Hirasa O, Yamauchi A (1989) Kobunshi Ronbunshu 46:427
Neugebauer D, Matyjaszewski K (2003) Macromolecules 36:2598
Chiefari J, Chong YKB, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, Rirrardo E, Thang SH (1998) Macromolecules 31:5559
Rizzardo E, Chiefari J, Mayadunne RTA, Moad G, Thang SH (1999) Polym Prepr Am Chem Soc Polym Chem Div 40:342
Donovan MS, Lowe AB, Sumerlin BS, McCormick CL (2002) Macromolecules 35:4123
Theis A, Feldermann A, Charton N, Stenzel MH, Davis TP, Barner-Kowollik C (2005) Macromolecules 38:2595
Li C, Benicewicz BC (2005) J Polym Sci A Polym Chem 43:1535
Barner-Kowollik C, Davis TP, Heuts JPA, Stenzel MH, Vana P, Whittaker M (2003) J Polym Sci A Polym Chem 41:365
Duréault A, Taton D, Destarac M, Leising F, Gnanou Y (2004) Macromolecules 37:5513
Moad G, Chiefari J, Chong YKB, Krstina J, Mayadunne RTA, Postma A, Rizzardo E, Thang SH (2000) Polym Int 49:993
Arita T, Buback M, Vana P (2005) Macromolecules 38:7935–7943
Feldermann A, Davis TP, Stenzel MH, Barner-Kowollik C (2005) Polymer 46:8448–8457
Lecommandoux S, Sandre O, Chécot F, Rodriguez-Hernandez J, Perzynski R (2005) Adv Mater 17:712
Kwon Y, Faust R, Chen CX, Thomas EL (2002) Macromolecules 35:3348
Bian K, Cunningham MF (2005) Macromolecules 38:695
Cheng CX, Huang Y, Tang RP, Chen E, Xi F (2005) Macromolecules 38:3044–3047
Park EK, Lee SB, Lee YM (2005) Biomaterials 26:1053
Talingting MR, Munk P, Webber SE, Tuzar Z (1999) Macromolecules 32:1593
Paz Banez de MV, Robinson KL, Armes SP (2000) Macromolecules 33:451
Wang X, Winnik MA, Manners I (2005) Macromolecules 38:1928
Chen X, Ding X, Zheng Z, Peng Y (2005) Macromol Biosci 5:157
Voulgaris D, Tsitsilianis C (2001) Macromol Chem Phys 202:3284
Schillen K, Yekta A, Ni S, Winnik MA (1998) Macromolecules 31:210
Torchilin VP (2001) J Control Release 73:137
Yusa S, Fukuda K, Yamamoto T, Ishihara K, Morishima Y (2005) Biomacromolecules 6:663
Pillay Narrainen A, Pascual S, Haddleton DM (2002) J Polym Sci A Polym Chem 40:439
Qin A, Tian M, Ramireddy C, Webber SE, Munk P (1994) Macromolecules 27:120
Antonietti M, Heinz S, Schmidt M, Rosenauer C (1994) Macromolecules 27:3276
Chécot F, Brûlet A, Oberdisse J, Gnanou Y, Mondain-Monval O, Lecommandoux S (2005) Langmuir 21:4308
Schuch H, Klingler J, Rossmanith P, Frechen T, Gerst M, Feldthusen J, Müller AHE (2000) Macromolecules 33:1734
Save M, Manguian M, Chassenieux C, Charleux B (2004) Macromolecules 38:280
Samii AA, Karlstrom G, Lindman B (1991) Langmuir 7:1067
Yang L, Alexandritis P (2000) Langmuir 16:4819
Huang W, Zhou Y, Yan D (2005) J Polym Sci A Polym Chem 43:2038
Bertin PA, Watson KJ, Nguyen S (2004) Macromolecules 37:8364
Acknowledgements
We gratefully acknowledge J. Storsberg for the proving the monomer M2 (Fraunhofer Institute for Applied Polymer Research IAP, Potsdam-Golm) and P. Hennaux (Universität Potsdam) for the providing the monomer M3, M. Heydenreich and E. Kleinpeter (Universität Potsdam) for help with NMR spectroscopy, and R. Sigel and S. Kubowicz (Max Planck Institute for Colloid and Interface Research, Potsdam-Golm) for help with SLS analysis. Financial support was provided by Fonds der Chemischen Industrie and the DFG (project La611/4-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Garnier, S., Laschewsky, A. Non-ionic amphiphilic block copolymers by RAFT-polymerization and their self-organization. Colloid Polym Sci 284, 1243–1254 (2006). https://doi.org/10.1007/s00396-006-1484-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-006-1484-9