Abstract
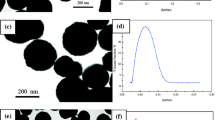
Magnetically loaded polymeric nano-particles carrying functional groups on their surface were prepared by a two-stage process. In the first stage, super-paramagnetic magnetite (Fe3O4) nano-particles were produced by a co-precipitation method from the aqueous solutions of FeCl2·4H2O and FeCl3·6H2O using a NaOH solution. The smallest size obtained was 40.9 nm with poly-dispersity index of 0.194 obtained by using a Zeta Sizer. The effects of Fe2+/Fe3+ molar ratio, stirring rate, temperature, base concentration, and pH on the particle size/size distribution and stability of the dispersions were examined. Increasing the relative concentration of Fe2+ ion and decreasing the stirring rate and pH increased the particle size, while the concentration of NaOH and temperature did not change the particle size significantly. Polymer coating was achieved by emulsion polymerization at high surfactant to monomer ratio of methyl methacrylate (MMA) and acrylic acid which were used as comonomers (comonomer ratio: 90/10 weight) with high surfactant to monomer ratio. The surfactant and initiator were SDS and KPS, respectively. Nano-particles in the range of 115 and 300 nm in diameter were produced depending on recipe. Increasing the Fe3O4/monomer and surfactant/monomer ratios, the KPS concentration caused a decrease in the average diameter. Magnetic properties of the nano-particles were obtained by electron spin resonance and vibrating-sample magnetometer. Most of the polymer-coated nano-particles exhibited super paramagnetic behavior.





Similar content being viewed by others

References
Yamaura M, Camilo RL, Sampaio LC, Macedo MA, Nakamura M, Toma HE (2004) J Magn Magn Mater 279:210–217
Ramirez LP, Landfester K (2003) Macromol Chem Phys 204:22–31
Šafařík I, Šafaříková M (2002) Monatsh Chem 133:737–759
Brigger I, Durernet C, Couvreur P (2002) Adv Drug Deliv Rev 54:631–651
Harma H (2002) Technol Rev 126:1–25
Deng Y, Wang L, Yang W, Fu S, Elaïssari A (2003) J Magn Magn Mater 257(1):69–78
Dresco PA, Zaitsev VS, Gambino RJ, Chu B (1999) Langmuir 15:1945–1951
Xu ZZ, Wang CC, Yang WL, Deng YH, Fu SK (2004) J Magn Magn Mater 277:136–143
Arias JL, Gallardo V, Gómez-Lopera SA, Plaza RC, Delgado AV (2001) J Controlled Release 77:309–321
Deng J, Peng Y, He C, Long X, Li P, Chan ASC (2003) Polym Int 52:1182–1187
Wormuth K (2001) J Colloid Interface Sci 241:366–377
Xie G, Zhang Q, Luo Z, Wu M, Li T(2003) J Appl Polym Sci 87:1733–1738
Zaitsev VS, Filimonov DS, Presnyakov IA, Gambino RJ, Chu B (1999) J Colloid Interface Sci 212:49–57
Babes L, Denizot B, Tanguy G, Jeune JJL, Jallet P (1999) J Colloid Interface Sci 212(2):474–482
Zhu Y, Wu Q (1999) J Nanopart Res 1:393–396
Massart R (1981) IEEE Trans Magn Mag 17:1247
Khalafalla SE, Reimers GW (1980) IEEE Trans Magn Mag 16:178
Pardoe H, Chua-anusorn W, St Pierre TG, Dobson J (2001) J Magn Magn Mater 225:41
Morais PC, Garg VK, Oliveira AC, Silva LP, Azevedo RB, Silva AML, Lima ECD (2001) J Magn Magn Mater 225:37–40
Gribanov NM, Bibik EE, Buzunov OV, Naumov VN (1990) J Magn Magn Mater 85:7
Liu ZL, Liu YJ, Yao KL, Ding ZH, Tao J, Wang X (2002) J Mater Synth Process 10:2
Kim DK, Zhang Y, Voit W, Rao KV, Muhammed M (2001) J Magn Magn Mater 225:30–36
Babes L, Denizot B, Tanguy G, Le Jeune JJ, Jallet P (1999) J Colloid Interface Sci 212:474
Jiang W, Yang HC, Yang SY, Horng HE, Hung JC, Chen YC, Hong CY (2004) J Magn Magn Mater (in press)
Horák D, Semenyuk N, Lednický F (2003) J Polym Sci Part A: Polym Chem 41:1848–1863
Cornell RM, Schertmann U (1991) Iron oxides in the laboratory; preparation and characterization. VCH Publishers, Weinheim
Bocanegra-Diaz A, Mohallem NDS, Sinisterra RD (2003) J Braz Chem Soc 14:936–941
Montagne F, Monval-Mondain O, Pichot C, Mozzanega H, Elaïssari A (2002) J Magn Magn Mater 250:302–312
Cornell RM, Schertmann U (1996) The iron oxides; structure, properties, reactions, occurrence and uses. VCH Publishers, Weinheim
Özer F, Beşkardeş MO, Zareie H, Pişkin E (2000) J Appl Polym Sci 82:237–242
Macias ER, Rodríguez-Guadarrama LA, Cisneros BA, Castañeda A, Mendizábal E, Puig JE (1995) Colloids Surf A: Physicochem Eng Aspects 103:119–126
Kondo A, Fukuda H (1999) Colloids Surf A: Physiochem Eng Aspects 153:435–438
David G, Özer F, Simionescu BC, Zareie H, Pişkin E (2002) Eur Polym J 38:73–78
Yildiz U, Capek I (2003) Polymer 44:2193–2200
Babaç C, Guven G, David G, Simionescu BC, Pişkin E (2004) Eur Polym J 40(8):1947–1952
Acknowledgement
Prof. Erhan Piskin was supported by Turkish Academy of Sciences as a full member.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00396-005-1426-y
Rights and permissions
About this article
Cite this article
Sayar, F., Güven, G. & Pişkin, E. Magnetically loaded poly(methyl methacrylate-co-acrylic acid) nano-particles. Colloid Polym Sci 284, 965–978 (2006). https://doi.org/10.1007/s00396-005-1383-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-005-1383-5