Abstract
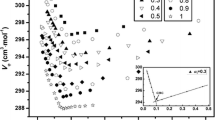
Two breaks have been found on the conductivity, refractive index, density and sound velocity against molality plots for aqueous solutions of dodecyldimethylbenzylammonium chloride at 25 °C, in the absence of any additive. The first break corresponds to the critical micelle concentration, cmc. The second, less distinct break, occurring in the molality range of 0.082 to 0.104 mol kg-1, in dependence on the technique applied, has been ascribed to the second critical micelle concentration, 2nd cmc, responsible for structural transitions of spherical micelles. Values of cmc and 2nd cmc have been also estimated conductometrically for tetradecyltrimethylammonium bromide and dodecylpyridinium chloride. On the basis of available conductometric data it has been shown that the 2nd cmc/cmc ratio varies in the range of 2 to 10 in dependence on the type of 1:1 ionic surfactant. It has been also shown that for a given class of surfactants, the logarithm of 2nd cmc varies linearly with the number of carbon atoms in the alkyl chain ( n= 12, 14 and 16). Both empirical regression coefficients depend upon the class of surfactants considered.




Similar content being viewed by others
References
Israelachvili J (1995) Intermolecular & Surface Forces. Academic Press, San Diego
Nagarajan R (2002) Langmuir 18:31
Miura M, Kodama M (1972) Bull Chem Soc Jpn 45:428
Ekwall P, Lemström KE, Eikrem H, Holmberg P (1967) Acta Chem Scand 21:1401
Reiss-Husson F, Luzzatti V (1964) J Phys Chem 88:3904
May S, Ben-Shaul A (2001) J Phys Chem B 105:640
Imae T, Ikeda S (1986) J Phys Chem 90:5216
Swanson-Vethamuthu M, Feitosa E, Brown W (1998) Langmuir 14:1590
Pisárcik M, Devínsky F, Svajdlenka E (1996) Colloids and Surfaces A 119:115
Zielinski R (1998) Polish J Chem 72:127
Miyagishi S (1976) Bull Chem Soc Jpn 49:34
Lianos P, Zana R (1984) J Colloid Interface Sci 101:587
Treiner C, Chattopadhyay AK, Bury R (1985) J Colloid Inteface Sci 104:569
Quirion F, Desnoyers JE (1987) J Colloid Interface Sci 115:176
Treiner C, Makayssi A (1992) Langmuir 8:794
Del Castillo JL, Czapkiewicz J, González-Pérez A, Rodríguez JR (2000) Colloids Surf A 166:161
González-Pérez A, Del Castillo JL, Czapkiewicz J, Rodríguez JR (2001) J Phys Chem B 105:1720
González-Pérez A, Czapkiewicz J, Del Castillo JL, Rodríguez JR (2001) Colloids Surf A 193:129
González-Pérez A, Del Castillo JL, Czapkiewicz J, Rodríguez JR (2002) Colloid Polym Sci 280:503
Okano LT, El Seoud OA, Halstead TK (1997) Colloid Polym Sci 275:138
Adderson JE, Taylor H (1971) J Pharm Pharmacol 23:311
Trompette JL, Zajac J, Keh E, Partyka S (1994) Langmuir 10:812
Makayssi A, Bury R, Treiner C (1994) Langmuir 10:1359
Monk CB (1961) Electrolytic Dissociation. Academic Press, London
De Lisi R, Fisicaro E, Milioto S (1988) J Sol Chem 17:1988
Stauff J (1938) Z Phys Chem A 183:55
Klevens HB (1953) J Am Chem Soc 30:74
Kopecki F (1996) Pharmazie 51:135
Chung MI, Tak IJ, Lee KM (1975) Taehan Hwahak Hoechi 19:398; (1976) CA 84 185293y
Hoffmann H, Rehage H, Platz G, Schorr W, Thurn H, Ulbricht W (1982) Colloid Polym Sci 260:1042
Acknowledgement
One of the authors, G-P, wishes to thank Prof. A. Amigo from the Department of Applied Physics, U. S. C., for enabling him to make refractive index measurements. This work received financial support from Xunta de Galicia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Pérez, A., Czapkiewicz, J., Prieto, G. et al. Second critical micelle concentration of dodecyldimethylbenzylammonium chloride in aqueous solution at 25 °C. Colloid Polym Sci 281, 1191–1195 (2003). https://doi.org/10.1007/s00396-003-0905-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-003-0905-2