Abstract
The effect of limb remote ischaemic conditioning (RIC) on myocardial infarct (MI) size and left ventricular ejection fraction (LVEF) was investigated in a pre-planned cardiovascular magnetic resonance (CMR) substudy of the CONDI-2/ERIC-PPCI trial. This single-blind multi-centre trial (7 sites in UK and Denmark) included 169 ST-segment elevation myocardial infarction (STEMI) patients who were already randomised to either control (n = 89) or limb RIC (n = 80) (4 × 5 min cycles of arm cuff inflations/deflations) prior to primary percutaneous coronary intervention. CMR was performed acutely and at 6 months. The primary endpoint was MI size on the 6 month CMR scan, expressed as median and interquartile range. In 110 patients with 6-month CMR data, limb RIC did not reduce MI size [RIC: 13.0 (5.1–17.1)% of LV mass; control: 11.1 (7.0–17.8)% of LV mass, P = 0.39], or LVEF, when compared to control. In 162 patients with acute CMR data, limb RIC had no effect on acute MI size, microvascular obstruction and LVEF when compared to control. In a subgroup of anterior STEMI patients, RIC was associated with lower incidence of microvascular obstruction and higher LVEF on the acute scan when compared with control, but this was not associated with an improvement in LVEF at 6 months. In summary, in this pre-planned CMR substudy of the CONDI-2/ERIC-PPCI trial, there was no evidence that limb RIC reduced MI size or improved LVEF at 6 months by CMR, findings which are consistent with the neutral effects of limb RIC on clinical outcomes reported in the main CONDI-2/ERIC-PPCI trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mortality and heart failure in ST-segment elevation myocardial infarction (STEMI) patients reperfused by primary percutaneous coronary intervention (PPCI) remain significant [38]. As such, new treatments that can be administered as adjuncts to PPCI, are needed to reduce myocardial infarct (MI) size, prevent adverse post-infarct left ventricular (LV) remodelling, and reduce the risk of developing heart failure (HF) [13, 21, 27, 29]. In this regard, remote ischaemic conditioning (RIC), in which brief cycles of non-lethal ischaemia and reperfusion are applied to an organ or tissue (including the arm or leg) away from the heart, has been shown to reduce MI size in small and large animal models of acute myocardial ischaemia/reperfusion injury (IRI) [11, 25, 28, 37]. In the clinical setting, the RIC stimulus can be non-invasively applied to the arm or leg using serial inflations and deflations (3–4 × 5 min cycles) of a pneumatic cuff placed on the upper arm or thigh, to induce brief non-lethal cycles of limb ischaemia and reperfusion [33]. Limb RIC has been demonstrated to increase myocardial salvage and reduce MI size by 20–30% (quantified by cardiac biomarkers, myocardial single-photon emission computerized tomography [SPECT] or cardiovascular magnetic resonance [CMR]), when applied as an adjunct to reperfusion in STEMI patients treated by either thrombolysis [49] or PPCI [1, 12, 46], although not all studies have shown a cardioprotective effect on MI size [15, 31, 44]. Furthermore, a few studies [18, 41, 42] have shown that RIC may improve clinical outcomes (death and rehospitalisation for heart failure) in STEMI patients undergoing PPCI, but they were not adequately powered for these hard clinical outcomes. However, the recently published large multi-centre CONDI-2/ERIC-PPCI trial (comprising 5401 STEMI patients), reported that limb RIC applied as an adjunct to PPCI did not reduce rates of cardiac death or rehospitalisation for HF at 12 months [22].
CMR has emerged as the imaging modality of choice for assessing cardioprotective efficacy of novel therapies for reducing MI size and preventing adverse post-infarct LV remodelling in STEMI, as it is the reference standard imaging modality for quantifying MI size, LV dimensions and function [3, 26, 36]. Furthermore, CMR is able to detect prognostically important coronary microvascular complications of acute myocardial IRI such as microvascular obstruction (MVO) and intramyocardial haemorrhage (IMH) [9].
In a pre-planned CMR substudy of the CONDI-2/ERIC-PPCI trial, we investigated the effect of limb RIC on acute and chronic MI size, MVO, IMH, and LV function and volumes. We hypothesized that limb RIC applied as an adjunct to PPCI would reduce MI size, prevent MVO and IMH, and improve LVEF. This would shed some light on whether the main CONDI-2/ERIC-PPCI trial [22] was neutral because of a lack of benefit of RIC to reduce MI size by CMR or whether there was an impact on MI size, which was not sufficient to translate to an improvement in clinical outcomes.
Methods
This was a pre-planned CMR substudy of the published CONDI-2/ERIC-PPCI trial [22]. The study received ethical approval from regional and National Health Service research ethics committees and was conducted in accordance with the principles of good clinical practice. In the CONDI-2 component of the study, all participants provided written informed consent before randomisation. In the ERIC-PPCI component of the study, all patients provided initial verbal assent before randomisation, which was followed by written informed consent. In the main CONDI-2/ERIC-PPCI trial, patients were randomised to receive either limb RIC or control [22]. Limb RIC was achieved using an automated pneumatic cuff (CellAegis AutoRIC, Toronto, Ontario, Canada) placed on the upper arm, and comprised of four alternating cycles of 5 min inflations to 200 mmHg and 5 min deflations to 0 mmHg. The control group comprised either a sham device (UK) or standard care (Denmark). RIC was initiated before PPCI either in the ambulance (Denmark) or on arrival at the hospital (UK), and the RIC or sham protocols did not delay onset of PPCI. Patient recruitment took place at seven CMR centres (five in the UK and two in Denmark). Patients in the seven CMR substudy centres were approached for participation in the CMR substudy. The London School of Hygiene & Tropical Medicine Clinical Trials Unit (London, UK) coordinated the trial in collaboration with the Cardiology Trial Unit and Department of Clinical Epidemiology of Aarhus University Hospital (Aarhus, Denmark).
Patient inclusion criteria in the CMR substudy was a diagnosis of STEMI and pre-PPCI TIMI flow ≤ 1. Exclusion criteria were contraindications to CMR scanning (ferromagnetic implants, estimated glomerular filtration rate < 30 ml/min/1.73m2) or inability to tolerate a CMR scan (e.g. claustrophobia, inability to lie flat or mechanical complications, hemodynamic instability due to ventricular arrhythmias or cardiogenic shock), previous coronary artery bypass graft surgery, myocardial infarction within the previous 30 days, left bundle branch block on ECG, treatment with therapeutic hypothermia, conditions precluding use of RIC (paresis of upper limb, or presence of an arteriovenous shunt), and life expectancy of less than 1 year due to non-cardiac pathology.
CMR image acquisition
There was a standardised CMR acquisition protocol in place prior to the start of the study and the CMR endpoints analysis were pre-defined prior to unblinding of any data. Patients underwent CMR scans acutely (aiming for days 3 post-PPCI and up to 7 days), and at 6 months post-PPCI. CMR was performed using the following scanners: Siemens 1.5T (Barts Heart Centre, Bristol Royal Infirmary, Royal Free Hospital in the UK, and Copenhagen Hospital in Denmark), Siemens 3.0T (John Radcliffe Hospital, UK), and Philips 1.5T (Leeds General Infirmary, UK and Aarhus Hospital, Denmark). The CMR protocol included cine images, full LV stack acquisitions of native T1, and basal, mid- and apical LV short-axis T2* maps, full LV stack of late gadolinium enhancement (LGE, 10 min following Gadovist bolus [0.1 mmol/kg]). All short-axis maps and LGE images were aligned with the short-axis cine images.
Study endpoints
The primary endpoint was MI size on the 6 months CMR scan (expressed as % of LV mass). Secondary endpoints included: acute MI size (expressed as % of LV mass), edema-based myocardial salvage index (edema-based area-at-risk quantified by the extent of myocardial edema on T1-maps minus MI size divided by the edema-based area-at-risk), incidence of MVO (on LGE imaging) and incidence of IMH (on T2* maps); LV volumes (LV end diastolic volume, LVEDV; LV end systolic volume, LVESV); LV mass in grams; and LV systolic function (LV ejection fraction [LVEF]).
CMR image analysis
The CMR images were uploaded to a secure server to enable transfer to the CMR core lab. CMR parameters were analyzed using dedicated software (CVI42, Circle Cardiovascular Imaging, Calgary, Canada). All images were analyzed by two experienced observers (RF and JC), and reviewed by a third experienced senior observer (HB). Our core lab has previously shown excellent inter-observer and intra-observer reproducibility for infarct size measurement [7]. All observers were blinded to the treatment allocation. LV volumes and function were quantified using disk summation method, with papillary muscles included as part of the LV cavity [4]. The AAR was quantified using native T1 mapping on the acute scan using the 2-SD semi-automated technique and MI size was quantified from the LGE images using the 5-SD semi-automated technique as previously described, expressed as the percentage of overall LV mass [5]. MVO was detected as dark cores within the bright areas of LGE and was included as part of the acute MI size and MVO was also quantified in a binary fashion as present or absent. IMH was identified as the hypointense areas within the infarct-related territory with T2* values < 20 ms on at least one of the short-axis T2* maps [6].
Statistical analysis
Statistical analysis was performed using commercial statistical software (Stata Statistical Software: Release 15.1. College Station, TX: StataCorp LLC) according to the pre-defined statistical analysis plan that was produced prior to any unblinding of the treatment codes. Continuous data were described as mean ± standard deviation (SD) or median (interquartile ranges [IQR]) as appropriate. Categorical data were described as frequencies and percentages. Groups were compared for continuous outcomes using linear regression methods using transformation of outcome data where the distribution was clearly non-normal with mean differences and 95% confidence intervals (CIs) presented. Where a suitable transformation could not be found, non-parametric methods using Mann–Whitney U test were used. Risk differences were calculated for binary outcomes together with 95% CIs. A post hoc analysis was undertaken in left anterior descending (LAD) STEMI patients.
Results
Baseline characteristics
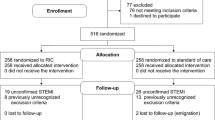
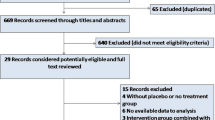
1264 patients were recruited at the 7 CMR sites, and of these 169 patients were recruited into the CMR substudy between 7 January 2016 and 26 March 2018, with 162 having analysable acute CMR scans performed at a median of 2 days post-PPCI (interquartile range 1–3 days). Of these, 110 completed the chronic scans performed at 6 months post-PPCI. The flowchart of the patients included in this CMR substudy is shown in Fig. 1, and representative coronary angiography and acute CMR images are provided for two STEMI patients in Fig. 2. The baseline characteristics and PPCI details were reasonably well-balanced between RIC and control (Table 1). Table 2 compares the baseline and procedural characteristics of the patients in the main study with those in the CMR substudy; those in the latter group were more likely to be younger, to be of male sex and more likely to present with RCA territory STEMI.
Representative coronary angiography and acute CMR images from 2 CONDI-2/ERIC-PPCI patients. Patient 1. A Pre- and post-PCI angiographic images showing a proximally occluded LAD artery, treated successfully by PPCI. B Short-axis T2*-maps showing areas of IMH in the anterior wall and septum. C Short-axis T1-maps revealing extensive myocardial oedema in the LAD territory with hypointense areas corresponding to areas of MVO and IMH. D Short-axis late gadolinium enhancement images showing large area of infarction in LAD territory with MVO. Patient 2. A Pre- and post-PCI angiographic images showing an occluded circumflex artery, treated successfully by PPCI. B Short-axis T2*-maps shows no evidence of IMH. C Short-axis T1-maps revealing myocardial oedema in the lateral wall with hypointense areas corresponding to areas of MVO. D Short-axis late gadolinium enhancement images showing a lateral infarct with MVO
Acute and chronic CMR endpoints
There was no impact of limb RIC on the primary endpoint of MI size on the 6 months CMR scan [RIC: 13.0 (5.1–17.1)% of LV mass; control: 11.1 (7.0–17.8)% of LV mass, P = 0.39, Fig. 3a, Table 3] when compared to control. There was also no difference in LVEF (Table 3), between the RIC group and control. Furthermore, there was also no difference in acute MI size between the RIC group and control (Fig. 3b, Table 3). RIC had no effect on the extent of the edema (Table 3) or LV ejection fraction (Table 3) on the acute CMR scan, when compared to control. There was no difference in the incidence of MVO (RIC 49%; control 61%, P = 0.13) and IMH (RIC 36%; control 52%, P = 0.067) between RIC and control.
LAD and non-LAD STEMI subgroups
In a post hoc analysis of 54 left anterior descending (LAD) STEMI patients, there remained no differences in acute (Table 4) and chronic MI size (Table 4), between the RIC group and control. Of note, LVEF on the acute scan was significantly higher in the RIC group [RIC 49 (42–52)%; control 42 (36–50)%; P = 0.041, Table 4], when compared to control. There was also an associated lower incidence of MVO in the RIC group (RIC 52%; control 83%, P = 0.012) when compared to control. However, this did not translate to an improvement in LVEF at 6 months (Table 4). There were no differences in the CMR parameters in the non-LAD STEMI subgroup as detailed in Table 4.
Discussion
In this pre-planned CMR substudy of the CONDI-2/ERIC-PPCI trial, limb RIC applied as an adjunct to PPCI had no observed beneficial effects on the primary endpoint of chronic MI size at 6 months post-PPCI, or the secondary endpoints of acute MI size, myocardial salvage index or LV ejection fraction when compared to control. Although RIC was associated with less MVO and better acute LVEF in the LAD STEMI subgroup, this did not translate to an improvement in LVEF as 6 months. Overall, these findings are consistent with the neutral effects of limb RIC on clinical outcomes reported in the main CONDI-2/ERIC-PPCI trial [22].
Our study findings do not support the results of previously published RIC studies in STEMI patients [1, 12, 18, 41, 42, 46]. Furthermore, in a subset of 2662 patients in the main CONDI-2/ERIC-PPCI trial, limb RIC did not reduce acute MI size quantified by the 48-h cardiac troponin area-under-the-curve, when compared to control [22] and this is consistent with our CMR substudy findings. However, it is worth noting that serial troponin data were incomplete in the majority of those 2662 patients in the main CONDI-2/ERIC-PPCI trial and there was no surrogate endpoint to account for the area-at-risk [30].
The potential benefit in acute LVEF and MVO seen in the LAD subgroup may have been due to the small sample size and type 1 error. However, there are some supporting evidence to suggest that those patients presenting with a large area-at-risk such as LAD-territory STEMI and with pre-PCI TIMI flow 0 or 1 are more likely to benefit from cardioprotective strategies that are initiated prior to reperfusion [8, 24, 30]. The translation of promising cardioprotective interventions from bench to bedside has proven challenging so far as laboratory experiments are conducted young animals with no comorbidities and focus on mechanistic insights (reductionist model) [40], whereas clinical trials are conducted in a heterogeneous cohort of patients with multiple comorbidities, taking concomitant medications and who may have varying degrees of pre-infarct angina, ischaemic time and anterograde and retrograde flow to the infarct-related territory at presentation. Real-world pragmatic studies have adopted an all-comer approach to facilitate recruitment in a timely fashion and to also make the promising cardioprotective therapy more widely applicable rather than adopting a more selective inclusion criteria of patients most likely to benefit. This approach may potentially dilute the effect size of the intervention [24] and may, in part, account for the neutral results of the CONDI-2/ERIC-PPCI trial [22].
Differences in the study design, patient cohorts recruited, the limb RIC protocol itself, and co-medications administered at the time of PPCI, may, in part, explain the discordant results of our CMR substudy with the published positive studies. Our study was a multi-centre trial across seven sites in UK and Denmark, whereas most of the previously published positive studies were single-centre studies, and may have been subjected to bias. We recruited all-comer STEMI patients with occluded coronary arteries, which may have diluted the cardioprotective effect of limb RIC, with prior studies reporting benefit in patients with larger LAD infarcts [1, 12]. However, two studies which recruited only anterior STEMI patients were also neutral [15, 44]. Furthermore, in the main CONDI-2/ERIC-PPCI trial, there was no difference in clinical outcomes with RIC in the LAD vs non-LAD subgroups. In our substudy, the limb RIC protocol comprised 4 × 5 min arm cuff inflations/deflation mainly delivered on arrival at the hospital, where in some cases, it overlapped with reperfusion, whereas in other studies limb RIC was applied in the ambulance and was completed prior to reperfusion, and may have, therefore, been more effective [1]. However, in the main CONDI-2/ERIC-PPCI trial, there was no difference in clinical outcomes whether limb RIC was delivered in the ambulance or at the hospital. Prior positive studies applied RIC to the leg [12, 18], which may have made RIC more effective given the greater tissue mass [35]. However, two studies applied RIC to the leg and were neutral [15, 44]. The majority of patients in our study were administered the oral P2Y12 inhibitor, ticagrelor prior to PPCI, whereas in the prior positive studies, clopidogrel was predominantly used [1, 12, 46]. Animal studies have reported ticagrelor to have cardioprotective effects [34, 45, 47, 48], and this may have attenuated the benefits of limb RIC in our CMR substudy. However, in the main CONDI-2/ERIC-PPCI trial, there was no difference in clinical outcomes with RIC, when stratified by whether ticagrelor was given or not. Finally, our patient cohort was a low-risk STEMI population comprising > 95% with patients in Killip class I, and our patients received timely and optimal treatment by PPCI, and this may have diminished the cardioprotective effects of RIC [20, 23, 30].
Some studies have shown additive cardioprotective benefits in terms of MI size reduction, increased myocardial salvage, and improve clinical outcomes when limb RIC was combined with other interventions such as morphine [39] or ischaemic postconditioning [14] suggesting that a multi-target approach using combination therapy may be more effective than administering a single cardioprotective intervention. The Remote Ischemic Conditioning With Local Ischemic Postconditioning in High-Risk ST-elevation Myocardial Infarction trial (RIP-HIGH, clinicaltrials.gov identifier: NCT04844931) will further explore this by combining both remote ischemic preconditioning with local ischemic post-conditioning in a high-risk (Killip class ≥ II) STEMI patient. The planned i-RIC trial will investigate the cardioprotective efficacy of a telehealth intervention to monitor compliance in real-time of similar intensive limb RIC protocol following STEMI [50]. Unfortunately, a recent study reported that applying daily episodes of limb RIC (4 × 5-min cycles on the arm) for 1 month, initiated on day 3 post-PPCI, did not reduce MI size or prevent adverse post-infarct LV remodelling at 4 months post-STEMI [43], although in the latter study commencing chronic limb RIC 3 days post-PPCI may have been too late to target key proponents of acute myocardial IRI, to prevent adverse post-infarct LV remodelling.
The main limitations of our study are, first, those in the CMR substudy were younger, more likely to be male and were more likely to present with RCA territory STEMI than those in the main trial. Second, the number of patients who completed the CMR scans was smaller than the planned sample size of 250 patients and, therefore, our study may be underpowered. An important number of patients did not undergo their chronic CMR, but we did not collect information on the specific reasons for these dropouts. The predominant reasons were likely due to a combination of patient-related and logistic reasons. These factors highlight the challenges of using CMR as a surrogate endpoint as only those able to tolerate a CMR scan and survive to 6 months would enter the substudy which creates an element of selection bias. The recent introduction of fast scanning CMR protocols for MI size and LVEF to under 15 min [19] may help to make CMR more tolerable to patients, keep costs down and improve accessibility, all of which may reduce dropout rates in future studies. We have presented data on the edema-based area-at-risk and edema-based myocardial salvage index in Tables 3 and 4 as this was a pre-planned secondary analysis in this substudy. However, recent data have shown that the extent of edema can be dynamic within the first few days of a STEMI [10, 16] and certain cardioprotective therapies that reduce MI size can also reduce the extent of the edema [2, 17]. As a result, the recent Journal of the American College of Cardiology scientific panel consensus document has advised against the use for the extent of edema as a surrogate for the area-at-risk in future studies [32].
In summary, in our pre-planned CMR substudy of the CONDI-2/ERIC-PPCI trial, we found that limb RIC did not reduce acute or chronic MI size or affect post-infarct LVEF, findings which are consistent with the neutral effects of limb RIC on clinical outcomes observed in the main CONDI-2/ERIC-PPCI trial [22]. Whether limb RIC may confer benefit in higher risk STEMI patients such as those presenting with cardiac arrest or cardiogenic shock, or in those countries in which ischaemic times are prolonged and PPCI is not widely available, remains to be tested [30].
References
Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sørensen HT, Redington AN, Nielsen TT (2010) Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375:727–734. https://doi.org/10.1016/S0140-6736(09)62001-8
Bulluck H, Chan M, Paradies V, Bryant J, Hernández-Reséndiz S, Cabrera-Fuentes H, Watson T, Chan M, Tan J, Hausenloy D (2018) Impact of cardioprotective therapies on the edema-based area at risk by CMR in reperfused STEMI. J Am Coll Cardiol 71:2856–2858. https://doi.org/10.1016/J.JACC.2018.04.016
Bulluck H, Dharmakumar R, Arai AE, Berry C, Hausenloy DJ (2018) Cardiovascular magnetic resonance in acute st-segment-elevation myocardial infarction: recent advances, controversies, and future directions. Circulation 137:1949–1964. https://doi.org/10.1161/CIRCULATIONAHA.117.030693
Bulluck H, Go YY, Crimi G, Ludman AJ, Rosmini S, Abdel-Gadir A, Bhuva AN, Treibel TA, Fontana M, Pica S, Raineri C, Sirker A, Herrey AS, Manisty C, Groves A, Moon JC, Hausenloy DJ (2017) Defining left ventricular remodeling following acute ST-segment elevation myocardial infarction using cardiovascular magnetic resonance. J Cardiovasc Magn Reson 19:1–13. https://doi.org/10.1186/s12968-017-0343-9
Bulluck H, Hammond-Haley M, Fontana M, Knight D, Sirker A, Herrey A, Manisty C, Kellman P, Moon J, Hausenloy D (2017) Quantification of both the area-at-risk and acute myocardial infarct size in ST-segment elevation myocardial infarction using T1-mapping. J Cardiovasc Magn Reson. https://doi.org/10.1186/S12968-017-0370-6
Bulluck H, Rosmini S, Abdel-Gadir A, Bhuva AN, Treibel TA, Fontana M, Gonzalez-Lopez E, Ramlall M, Hamarneh A, Sirker A, Herrey AS, Manisty C, Yellon DM, Moon JC, Hausenloy DJ (2017) Diagnostic performance of T1 and T2 mapping to detect intramyocardial hemorrhage in reperfused ST-segment elevation myocardial infarction (STEMI) patients. J Magn Reson Imaging 46:877–886. https://doi.org/10.1002/jmri.25638
Bulluck H, Rosmini S, Abdel-Gadir A, Bhuva AN, Treibel TA, Fontana M, Weinmann S, Sirker A, Herrey AS, Manisty C, Moon JC, Hausenloy DJ (2016) Impact of microvascular obstruction on semiautomated techniques for quantifying acute and chronic myocardial infarction by cardiovascular magnetic resonance. Open Heart 3:e000535. https://doi.org/10.1136/openhrt-2016-000535
Bulluck H, Yellon D, Hausenloy D (2016) Reducing myocardial infarct size: challenges and future opportunities. Heart 102:341–348. https://doi.org/10.1136/HEARTJNL-2015-307855
Carrick D, Haig C, Ahmed N, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay MM, Davie A, Mahrous A, Mordi I, Rauhalammi S, Sattar N, Welsh P, Radjenovic A, Ford I, Oldroyd KG, Berry C (2016) Myocardial hemorrhage after acute reperfused ST-segment-elevation myocardial infarction: relation to microvascular obstruction and prognostic significance. Circ Cardiovasc Imaging 9:e004148. https://doi.org/10.1161/CIRCIMAGING.115.004148
Carrick D, Haig C, Ahmed N, Rauhalammi S, Clerfond G, Carberry J, Mordi I, McEntegart M, Petrie M, Eteiba H, Hood S, Watkins S, Lindsay M, Mahrous A, Welsh P, Sattar N, Ford I, Oldroyd K, Radjenovic A, Berry C (2016) Temporal evolution of myocardial hemorrhage and edema in patients after acute ST-segment elevation myocardial infarction: pathophysiological insights and clinical implications. J Am Heart Assoc. https://doi.org/10.1161/JAHA.115.002834
Chong J, Bulluck H, Yap EP, Ho AF, Boisvert WA, Hausenloy DJ (2018) Remote ischemic conditioning in ST-segment elevation myocardial infarction—an update. Cond Med 1:13–22 (PMID: 30338313)
Crimi G, Pica S, Raineri C, Bramucci E, De Ferrari GM, Klersy C, Ferlini M, Marinoni B, Repetto A, Romeo M, Rosti V, Massa M, Raisaro A, Leonardi S, Rubartelli P, Oltrona Visconti L, Ferrario M (2013) Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACC Cardiovasc Interv 6:1055–1063. https://doi.org/10.1016/j.jcin.2013.05.011
Davidson SM, Ferdinandy P, Andreadou I, Bøtker HE, Heusch G, Ibáñez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, Garcia-Dorado D (2019) Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol 73:89–99. https://doi.org/10.1016/j.jcin.2014.05.015
Eitel I, Stiermaier T, Rommel KP, Fuernau G, Sandri M, Mangner N, Linke A, Erbs S, Lurz P, Boudriot E, Mende M, Desch S, Schuler G, Thiele H (2015) Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Hear J 36:3049–3057. https://doi.org/10.1093/eurheartj/ehv463
Elbadawi A, Awad O, Raymond R, Badran H, Mostafa AE, Saad M (2017) Impact of remote ischemic postconditioning during primary percutaneous coronary intervention on left ventricular remodeling after anterior wall ST-segment elevation myocardial infarction: a single-center experience. Int J Angiol 26:241–248. https://doi.org/10.1055/s-0037-1601870
Fernández-Jiménez R, Barreiro-Pérez M, Martin-García A, Sánchez-González J, Agüero J, Galán-Arriola C, García-Prieto J, Díaz-Pelaez E, Vara P, Martinez I, Zamarro I, Garde B, Sanz J, Fuster V, Sánchez P, Ibanez B (2017) Dynamic edematous response of the human heart to myocardial infarction: implications for assessing myocardial area at risk and salvage. Circulation 136:1288–1300. https://doi.org/10.1161/CIRCULATIONAHA.116.025582
Fernández-Jiménez R, Galán-Arriola C, Sánchez-González J, Agüero J, López-Martín G, Gomez-Talavera S, Garcia-Prieto J, Benn A, Molina-Iracheta A, Barreiro-Pérez M, Martin-García A, García-Lunar I, Pizarro G, Sanz J, Sánchez P, Fuster V, Ibanez B (2017) Effect of ischemia duration and protective interventions on the temporal dynamics of tissue composition after myocardial infarction. Circ Res 121:439–450. https://doi.org/10.1161/CIRCRESAHA.117.310901
Gaspar A, Lourenço AP, Pereira MÁ, Azevedo P, Roncon-Albuquerque R, Marques J, Leite-Moreira AF (2018) Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res Cardiol. https://doi.org/10.1007/s00395-018-0672-3
Gómez-Talavera S, Fernandez-Jimenez R, Fuster V, Nothnagel ND, Kouwenhoven M, Clemence M, García-Lunar I, Gómez-Rubín MC, Navarro F, Pérez-Asenjo B, Fernández-Friera L, Calero MJ, Orejas M, Cabrera JA, Desco M, Pizarro G, Ibáñez B, Sánchez-González J (2021) Clinical validation of a 3-dimensional ultrafast cardiac magnetic resonance protocol including single breath-hold 3-dimensional sequences. JACC Cardiovasc Imaging. https://doi.org/10.1016/J.JCMG.2021.02.031
Hausenloy DJ, Bøtker HE (2019) Why did remote ischaemic conditioning not improve clinical outcomes in acute myocardial infarction in the CONDI-2/ERIC-PPCI trial? Cardiovasc Res 115:e161–e163. https://doi.org/10.1093/cvr/cvz242
Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM, Garcia-Dorado D (2017) Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J 38:935–941. https://doi.org/10.1093/cvr/cvz242
Hausenloy DJ, Kharbanda RK, Møller UK, Ramlall M, Aarøe J, Butler R, Bulluck H, Clayton T, Dana A, Dodd M, Engstrom T, Evans R, Lassen JF, Christensen EF, Garcia-Ruiz JM, Gorog DA, Hjort J, Houghton RF, Ibanez B, Knight R, Lippert FK, Lønborg JT, Maeng M, Milasinovic D, More R, Nicholas JM, Jensen LO, Perkins A, Radovanovic N, Rakhit RD, Ravkilde J, Ryding AD, Schmidt MR, Riddervold IS, Sørensen HT, Stankovic G, Varma M, Webb I, Terkelsen CJ, Greenwood JP, Yellon DM, Bøtker HE (2019) Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): a single-blind randomised controlled trial. Lancet 394:1415–1424. https://doi.org/10.1016/S0140-6736(19)32039-2
Hausenloy DJ, Ntsekhe M, Yellon DM (2020) A future for remote ischaemic conditioning in high-risk patients. Basic Res Cardiol 115:35. https://doi.org/10.1007/s00395-020-0794-2
Heusch G (2017) Critical issues for the translation of cardioprotection. Circ Res 120:1477–1486. https://doi.org/10.1161/CIRCRESAHA.117.310820
Heusch G (2018) 25 years of remote ischemic conditioning: from laboratory curiosity to clinical outcome. Basic Res Cardiol 1133(113):1–4. https://doi.org/10.1007/S00395-018-0673-2
Heusch G (2019) Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol. https://doi.org/10.1007/S00395-019-0756-8
Heusch G (2020) Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 1712(17):773–789. https://doi.org/10.1038/s41569-020-0403-y
Heusch G, Bøtker H, Przyklenk K, Redington A, Yellon D (2015) Remote ischemic conditioning. J Am Coll Cardiol 65:177–195. https://doi.org/10.1016/J.JACC.2014.10.031
Heusch G, Gersh B (2017) The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J 38:774–784. https://doi.org/10.1093/EURHEARTJ/EHW224
Heusch G, Gersh BJ (2020) Is cardioprotection salvageable? Circulation 141:415–417. https://doi.org/10.1161/CIRCULATIONAHA.119.044176
Heusch G, Rassaf T (2016) Time to give up on cardioprotection? a critical appraisal of clinical studies on ischemic pre-, post-, and remote conditioning. Circ Res 119:676–695. https://doi.org/10.1161/CIRCRESAHA.116.308736
Ibanez B, Aletras A, Arai A, Arheden H, Bax J, Berry C, Bucciarelli-Ducci C, Croisille P, Dall’Armellina E, Dharmakumar R, Eitel I, Fernández-Jiménez R, Friedrich M, García-Dorado DA, Hausenloy D, Kim R, Kozerke S, Kramer C, Salerno M, Sánchez-González J, Sanz J, Fuster V (2019) Cardiac MRI endpoints in myocardial infarction experimental and clinical trials: JACC scientific expert panel. J Am Coll Cardiol 74:238–256. https://doi.org/10.1016/J.JACC.2019.05.024
Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R (2002) Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106:2881–2883. https://doi.org/10.1161/01.CIR.0000043806.51912.9B
Kleinbongard P, Andreadou I, Vilahur G (2021) The platelet paradox of injury versus protection in myocardial infarction—has it been overlooked? Basic Res Cardiol 116:37. https://doi.org/10.1007/S00395-021-00876-6
Lieder HR, Irmert A, Kamler M, Heusch G, Kleinbongard P (2019) Sex is no determinant of cardioprotection by ischemic preconditioning in rats, but ischemic/reperfused tissue mass is for remote ischemic preconditioning. Physiol Rep. https://doi.org/10.14814/phy2.14146
Niccoli G, Montone RA, Ibanez B, Thiele H, Crea F, Heusch G, Bulluck H, Hausenloy DJ, Berry C, Stiermaier T, Camici PG, Eitel I (2019) Optimized treatment of ST-elevation myocardial infarction. Circ Res 125:245–258. https://doi.org/10.1161/CIRCRESAHA.119.315344
Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P (1993) Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87:893–899. https://doi.org/10.1161/01.cir.87.3.893
Puymirat E, Cayla G, Cottin Y, Elbaz M, Henry P, Gerbaud E, Lemesle G, Popovic B, Labèque JN, Roubille F, Andrieu S, Farah B, Schiele F, Ferrières J, Simon T, Danchin N (2019) Twenty-year trends in profile, management and outcomes of patients with ST-segment elevation myocardial infarction according to use of reperfusion therapy: Data from the FAST-MI program 1995–2015. Am Heart J 214:97–106. https://doi.org/10.1016/j.ahj.2019.05.007
Rentoukas I, Giannopoulos G, Kaoukis A, Kossyvakis C, Raisakis K, Driva M, Panagopoulou V, Tsarouchas K, Vavetsi S, Pyrgakis V, Deftereos S (2010) Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. JACC Cardiovasc Interv 3:49–55. https://doi.org/10.1016/j.jcin.2009.10.015
Rossello X, Yellon D (2016) Cardioprotection: the disconnect between bench and bedside. Circulation 134:574–575. https://doi.org/10.1161/CIRCULATIONAHA.116.022829
Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sørensen HT, Bøtker HE (2014) Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 35:168–175. https://doi.org/10.1093/eurheartj/eht369
Stiermaier T, Jensen JO, Rommel KP, De Waha-Thiele S, Fuernau G, Desch S, Thiele H, Eitel I (2019) Combined intrahospital remote ischemic perconditioning and postconditioning improves clinical outcome in st-elevation myocardial infarction: long-term results of the lipsia conditioning trial. Circ Res 124:1482–1491. https://doi.org/10.1161/CIRCRESAHA.118.314500
Vanezis AP, Arnold JR, Rodrigo G, Lai FY, Debiec R, Nazir S, Khan JN, Ng LL, Chitkara K, Coghlan JG, Hetherington SL, McCann GP, Samani NJ (2018) Daily remote ischaemic conditioning following acute myocardial infarction: a randomised controlled trial. Heart 104:1955–1962. https://doi.org/10.1136/heartjnl-2018-313091
Verouhis D, Sörensson P, Gourine A, Henareh L, Persson J, Saleh N, Settergren M, Sundqvist M, Tornvall P, Witt N, Böhm F, Pernow J (2016) Effect of remote ischemic conditioning on infarct size in patients with anterior ST-elevation myocardial infarction. Am Heart J 181:66–73. https://doi.org/10.1016/j.ahj.2016.08.004
Vilahur G, Gutiérrez M, Casani L, Varela L, Capdevila A, Pons-Lladó G, Carreras F, Carlsson L, Hidalgo A, Badimon L (2016) Protective effects of ticagrelor on myocardial injury after infarction. Circulation 134:1708–1719. https://doi.org/10.1161/CIRCULATIONAHA.116.024014
White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, Tehrani S, Flett AS, Meier P, Ariti C, Davies JR, Moon JC, Yellon DM, Hausenloy DJ (2014) Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 8:178–188. https://doi.org/10.1016/j.jcin.2014.05.015
Yang X, Liu Y, Cui L, Yang X, Downey JM, Cohen MV (2013) Two classes of anti-platelet drugs reduce anatomical infarct size in monkey hearts. Cardiovasc Drug Therapy 27:109–115. https://doi.org/10.1007/s10557-012-6436-7
Yang XM, Liu Y, Cui L, Yang X, Liu Y, Tandon N, Kambayashi J, Downey JM, Cohen MV (2013) Platelet P2Y(1)(2) blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther 18:251–262. https://doi.org/10.1177/1074248412467692
Yellon DM, Ackbarkhan AK, Balgobin V, Bulluck H, Deelchand A, Dhuny MR, Domah N, Gaoneadry D, Jagessur RK, Joonas N, Kowlessur S, Lutchoo J, Nicholas JM, Pauvaday K, Shamloll O, Walker JM, Hausenloy DJ (2015) Remote ischemic conditioning reduces myocardial infarct size in STEMI patients treated by thrombolysis. J Am Coll Cardiol 65:2764–2765. https://doi.org/10.1016/j.jacc.2015.02.082
Zheng Y, Reinhardt JD, Li J, Hu D, Lin S, Wang L, Dai R, Fan Z, Ding R, Chen L, Yuan L, Xu Z, Cheng Y, Yan C, Zhang X, Wang L, Zhang X, Teng M, Yu Q, Yin A, Lu X (2020) Can clinical and functional outcomes be improved with an intelligent “internet plus”-based full disease cycle remote ischemic conditioning program in acute ST-elevation myocardial infarction patients undergoing percutaneous coronary intervention? Rationale and design of the i-RIC trial. Cardiovasc Drugs Ther. https://doi.org/10.1007/s10557-020-07022-9
Acknowledgements
We thank all patients for participating in this study and all study personnel for their invaluable assistance.
Funding
The ERIC-PPCI trial was funded by a British Heart Foundation Clinical Study Grant (CS/14/3/31002) and a University College London Hospital/University College London Biomedical Research Clinical Research grant. The CONDI-2 trial was funded by Danish Innovation Foundation grants (11-108354 and 11-115818), Novo Nordisk Foundation (NNF13OC0007447), and Trygfonden (109624). DJH was supported by the British Heart Foundation (FS/10/039/28270), National Institute for Health Research University College London Hospitals Biomedical Research Centre, Duke-National University Singapore Medical School, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and its Collaborative Centre Grant scheme (NMRC/CGAug16C006). HEB was supported by the Novo Nordisk Foundation (NNF14OC0013337, NNF15OC0016674). WYK was supported by the Health Research Fund of Central Denmark Region (A1000). VMF acknowledges support from the British Heart Foundation (BHF), the BHF Centre of Research Excellence Oxford, and the NIHR Oxford BRC. TE was supported by the Novo Nordisk Foundation and the Alfred Benzon Foundation. This article is based upon the work of COST Action EU-CARDIOPROTECTION (CA16225) and supported by COST (European Cooperation in Science and Technology).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All other authors declare no conflicts of interests or disclosures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Francis, R., Chong, J., Ramlall, M. et al. Effect of remote ischaemic conditioning on infarct size and remodelling in ST-segment elevation myocardial infarction patients: the CONDI-2/ERIC-PPCI CMR substudy. Basic Res Cardiol 116, 59 (2021). https://doi.org/10.1007/s00395-021-00896-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-021-00896-2