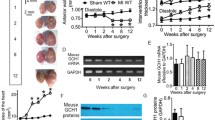
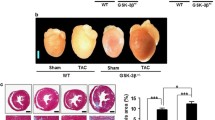
Abstract
Inhibition of the Ca2+-dependent proteases calpains attenuates post-infarction remodeling and heart failure. Recent data suggest that calpain activity is elevated in non-ischemic cardiomyopathies and that upregulation of the key cardiac G-protein-coupled receptor kinase 2 (GRK2) signaling hub promotes cardiac hypertrophy. However, the functional interactions between calpains and GRK2 in this context have not been explored. We hypothesized that calpain modulates GRK2 levels in myocardial hypertrophy of non-ischemic cause, and analyzed the mechanisms involved and the potential therapeutic benefit of inhibiting calpain activity in this situation. The oral calpain inhibitor SNJ-1945 was administered daily to male Sprague–Dawley rats or wild-type and hemizygous GRK2 mice treated with 5 mg/Kg/day isoproterenol intraperitoneally for 1 week. In isoproterenol-treated animals, calpains 1 and 2 were overexpressed in myocardium and correlated with increased calpain activity and ventricular hypertrophy. Oral co-administration of SNJ-1945 attenuated calpain activation and reduced heart hypertrophy as assessed using morphological and biochemical markers. Calpain activation induced by isoproterenol increased GRK2 protein levels, while genetic downregulation of GRK2 expression prevented isoproterenol-mediated hypertrophy independently of calpain inhibition. GRK2 upregulation was associated to calpain-dependent degradation of the GRK2 ubiquitin ligase MDM2 and to enhanced NF-κB-dependent GRK2 gene expression in correlation with calpain-mediated IĸB proteolysis. These results demonstrate that calpain mediates isoproterenol-induced myocardial hypertrophy by modulating GRK2 protein content through mechanisms involving the control of GRK2 stability and expression. Sustained calpain inhibition attenuates isoproterenol-induced myocardial hypertrophy and could be an effective therapeutic strategy to limit ventricular remodeling of non-ischemic origin.








Similar content being viewed by others
References
Arthur GD, Belcastro AN (1997) A calcium stimulated cysteine protease involved in isoproterenol induced cardiac hypertrophy. Mol Cell Biochem 176:241–248
Bermejo M, Martin-Serrano J, Oberlin E, Pedraza MA, Serrano A, Santiago B, Caruz A, Loetscher P, Baggiolini M, Arenzana-Seisdedos F, Alcami J (1998) Activation of blood T lymphocytes down-regulates CXCR4 expression and interferes with propagation of X4 HIV strains. Eur J Immunol 28:3192–3204. https://doi.org/10.1002/(SICI)1521-4141(199810)28:10%3c3192:AID-IMMU3192%3e3.0.CO;2-E
Bhuiyan MS, Shioda N, Fukunaga K (2009) Chronic beta-AR activation-induced calpain activation and impaired eNOS-Akt signaling mediates cardiac injury in ovariectomized female rats. Expert Opin Ther Targets 13:275–286. https://doi.org/10.1517/14728220902721312
Cao T, Fan S, Zheng D, Wang G, Yu Y, Chen R, Song LS, Fan GC, Zhang Z, Peng T (2019) Increased calpain-1 in mitochondria induces dilated heart failure in mice: role of mitochondrial superoxide anion. Basic Res Cardiol 114:17. https://doi.org/10.1007/s00395-019-0726-1
Freund C, Schmidt-Ullrich R, Baurand A, Dunger S, Schneider W, Loser P, El-Jamali A, Dietz R, Scheidereit C, Bergmann MW (2005) Requirement of nuclear factor-kappaB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation 111:2319–2325. https://doi.org/10.1161/01.CIR.0000164237.58200.5A
Galvez AS, Diwan A, Odley AM, Hahn HS, Osinska H, Melendez JG, Robbins J, Lynch RA, Marreez Y, Dorn GW 2nd (2007) Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ Res 100:1071–1078. https://doi.org/10.1161/01.RES.0000261938.28365.11
Garcia-Higuera I, Penela P, Murga C, Egea G, Bonay P, Benovic JL, Mayor F Jr (1994) Association of the regulatory beta-adrenergic receptor kinase with rat liver microsomal membranes. J Biol Chem 269:1348–1355. https://doi.org/10.1074/jbc.271.2.985
Gelis C, Mavon A, Vicendo P (2005) The contribution of calpains in the down-regulation of Mdm2 and p53 proteolysis in reconstructed human epidermis in response to solar irradiation. Photochem Photobiol 81:975–982. https://doi.org/10.1562/2004-08-05-RA-262
Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol Rev 83:731–801. https://doi.org/10.1152/physrev.00029.2002
Gupta S, Young D, Maitra RK, Gupta A, Popovic ZB, Yong SL, Mahajan A, Wang Q, Sen S (2008) Prevention of cardiac hypertrophy and heart failure by silencing of NF-κB. J Mol Biol 375:637–649. https://doi.org/10.1016/j.jmb.2007.10.006
Hernando V, Inserte J, Sartorio CL, Parra VM, Poncelas-Nozal M, Garcia-Dorado D (2010) Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J Mol Cell Cardiol 49:271–279
Hullmann J, Traynham CJ, Coleman RC, Koch WJ (2016) The expanding GRK interactome: implications in cardiovascular disease and potential for therapeutic development. Pharmacol Res 110:52–64. https://doi.org/10.1016/j.phrs.2016.05.008
Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ (2005) Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J 26:1752–1758. https://doi.org/10.1093/eurheartj/ehi429
Inserte J (2012) Calpains in the cardiovascular system. Cardiovasc Res 96:9–10. https://doi.org/10.1093/cvr/cvs245
Inuzuka H, Tseng A, Gao D, Zhai B, Zhang Q, Shaik S, Wan L, Ang XL, Mock C, Yin H, Stommel JM, Gygi S, Lahav G, Asara J, Xiao ZX, Kaelin WG Jr, Harper JW, Wei W (2010) Phosphorylation by casein kinase I promotes the turnover of the Mdm2 oncoprotein via the SCF(beta-TRCP) ubiquitin ligase. Cancer Cell 18:147–159. https://doi.org/10.1016/j.ccr.2010.06.015
Islam KN, Koch WJ (2012) Involvement of nuclear factor kappaB (NF-κB) signaling pathway in regulation of cardiac G protein-coupled receptor kinase 5 (GRK5) expression. J Biol Chem 287:12771–12778. https://doi.org/10.1074/jbc.M111.324566
Jean-Charles PY, Yu SM, Abraham D, Kommaddi RP, Mao L, Strachan RT, Zhang ZS, Bowles DE, Brian L, Stiber JA, Jones SN, Koch WJ, Rockman HA, Shenoy SK (2017) Mdm2 regulates cardiac contractility by inhibiting GRK2-mediated desensitization of beta-adrenergic receptor signaling. JCI Insight. https://doi.org/10.1172/jci.insight.95998
Karni-Schmidt O, Lokshin M, Prives C (2016) The Roles of MDM2 and MDMX in cancer. Annu Rev Pathol 11:617–644. https://doi.org/10.1146/annurev-pathol-012414-040349
Kawano S, Kubota T, Monden Y, Kawamura N, Tsutsui H, Takeshita A, Sunagawa K (2005) Blockade of NF-κB ameliorates myocardial hypertrophy in response to chronic infusion of angiotensin II. Cardiovasc Res 67:689–698. https://doi.org/10.1016/j.cardiores.2005.04.030
Kudo-Sakamoto Y, Akazawa H, Ito K, Takano J, Yano M, Yabumoto C, Naito AT, Oka T, Lee JK, Sakata Y, Suzuki J, Saido TC, Komuro I (2014) Calpain-dependent cleavage of N-cadherin is involved in the progression of post-myocardial infarction remodeling. J Biol Chem 289:19408–19419. https://doi.org/10.1074/jbc.M114.567206
Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L (2008) Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res 102:720–728. https://doi.org/10.1161/CIRCRESAHA.107.160077
Letavernier E, Zafrani L, Perez J, Letavernier B, Haymann JP, Baud L (2012) The role of calpains in myocardial remodelling and heart failure. Cardiovasc Res 96:38–45. https://doi.org/10.1093/cvr/cvs099
Lohse MJ, Engelhardt S, Eschenhagen T (2003) What is the role of beta-adrenergic signaling in heart failure? Circ Res 93:896–906. https://doi.org/10.1161/01.RES.0000102042.83024.CA
Lombardi MS, Kavelaars A, Penela P, Scholtens EJ, Roccio M, Schmidt RE, Schedlowski M, Mayor F, Jr., Heijnen CJ (2002) Oxidative stress decreases G protein-coupled receptor kinase 2 in lymphocytes via a calpain-dependent mechanism. Mol Pharmacol 62:379–388
Lucas E, Jurado-Pueyo M, Fortuno MA, Fernandez-Veledo S, Vila-Bedmar R, Jimenez-Borreguero LJ, Lazcano JJ, Gao E, Gomez-Ambrosi J, Fruhbeck G, Koch WJ, Diez J, Mayor F Jr, Murga C (2014) Downregulation of G protein-coupled receptor kinase 2 levels enhances cardiac insulin sensitivity and switches on cardioprotective gene expression patterns. Biochim Biophys Acta 1842:2448–2456. https://doi.org/10.1016/j.bbadis.2014.09.004
Ma J, Wei M, Wang Q, Li J, Wang H, Liu W, Lacefield JC, Greer PA, Karmazyn M, Fan GC, Peng T (2012) Deficiency of Capn4 gene inhibits nuclear factor-kappaB (NF-κB) protein signaling/inflammation and reduces remodeling after myocardial infarction. J Biol Chem 287:27480–27489. https://doi.org/10.1074/jbc.M112.358929
Martinez-Martinez S, Gomez del Arco P, Armesilla AL, Aramburu J, Luo C, Rao A, Redondo JM (1997) Blockade of T cell activation by dithiocarbamates involves novel mechanisms of inhibition of nuclear factor of activated T cells. Mol Cell Biol 17:6437–6447. https://doi.org/10.1128/MCB.17.11.6437
Mayor F Jr, Cruces-Sande M, Arcones AC, Vila-Bedmar R, Briones AM, Salaices M, Murga C (2018) G protein-coupled receptor kinase 2 (GRK2) as an integrative signalling node in the regulation of cardiovascular function and metabolic homeostasis. Cell Signal 41:25–32. https://doi.org/10.1016/j.cellsig.2017.04.002
Murga C, Penela P, Zafra F, Mayor F Jr (1998) The subcellular and cellular distribution of G protein-coupled receptor kinase 2 in rat brain. Neuroscience 87:631–637. https://doi.org/10.1016/S0306-4522(98)00145-6
Oka T, Walkup RD, Tamada Y, Nakajima E, Tochigi A, Shearer TR, Azuma M (2006) Amelioration of retinal degeneration and proteolysis in acute ocular hypertensive rats by calpain inhibitor ((1S)-1-((((1S)-1-benzyl-3-cyclopropylamino-2,3-di-oxopropyl)amino)carbonyl)-3-me thylbutyl)carbamic acid 5-methoxy-3-oxapentyl ester. Neuroscience 141:2139–2145. https://doi.org/10.1016/j.neuroscience.2006.05.060
Patterson C, Portbury AL, Schisler JC, Willis MS (2011) Tear me down: role of calpain in the development of cardiac ventricular hypertrophy. Circ Res 109:453–462. https://doi.org/10.1161/CIRCRESAHA.110.239749
Penela P (2016) Chapter Three—ubiquitination and protein turnover of G-protein-coupled receptor kinases in GPCR signaling and cellular regulation. Prog Mol Biol Transl Sci 141:85–140. https://doi.org/10.1016/bs.pmbts.2016.04.002
Penela P, Ruiz-Gomez A, Castano JG, Mayor F Jr (1998) Degradation of the G protein-coupled receptor kinase 2 by the proteasome pathway. J Biol Chem 273:35238–35244. https://doi.org/10.1074/jbc.273.52.35238
Poncelas M, Inserte J, Aluja D, Hernando V, Vilardosa U, Garcia-Dorado D (2017) Delayed, oral pharmacological inhibition of calpains attenuates adverse post-infarction remodelling. Cardiovasc Res 113:950–961. https://doi.org/10.1093/cvr/cvx073
Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G (2014) Heart failure: preventing disease and death worldwide. ESC Heart Fail 1:4–25. https://doi.org/10.1002/ehf2.12005
Prado FP, Dos Santos DO, Blefari V, Silva CA, Machado J, Kettelhut IDC, Ramos SG, Baruffi MD, Salgado HC, Prado CM (2017) Early dystrophin loss is coincident with the transition of compensated cardiac hypertrophy to heart failure. PLoS One 12:e0189469. https://doi.org/10.1371/journal.pone.0189469
Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW 2nd, Koch WJ (2008) G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res 103:413–422. https://doi.org/10.1161/CIRCRESAHA.107.168336
Ramos-Ruiz R, Penela P, Penn RB, Mayor F Jr (2000) Analysis of the human G protein-coupled receptor kinase 2 (GRK2) gene promoter: regulation by signal transduction systems in aortic smooth muscle cells. Circulation 101:2083–2089. https://doi.org/10.1161/01.CIR.101.17.2083
Rengo G, Pagano G, Filardi PP, Femminella GD, Parisi V, Cannavo A, Liccardo D, Komici K, Gambino G, D’Amico ML, de Lucia C, Paolillo S, Trimarco B, Vitale DF, Ferrara N, Koch WJ, Leosco D (2016) Prognostic value of lymphocyte G protein-coupled receptor kinase-2 protein levels in patients with heart failure. Circ Res 118:1116–1124. https://doi.org/10.1161/CIRCRESAHA.115.308207
Salcedo A, Mayor F Jr, Penela P (2006) Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. EMBO J 25:4752–4762. https://doi.org/10.1038/sj.emboj.7601351
Saleem N, Prasad A, Goswami SK (2017) Apocynin prevents isoproterenol-induced cardiac hypertrophy in rat. Mol Cell Biochem. https://doi.org/10.1007/s11010-017-3253-0
Schlegel P, Reinkober J, Meinhardt E, Tscheschner H, Gao E, Schumacher SM, Yuan A, Backs J, Most P, Wieland T, Koch WJ, Katus HA, Raake PW (2017) G protein-coupled receptor kinase 2 promotes cardiac hypertrophy. PLoS One 12:e0182110. https://doi.org/10.1371/journal.pone.0182110
Sorriento D, Santulli G, Franco A, Cipolletta E, Napolitano L, Gambardella J, Gomez-Monterrey I, Campiglia P, Trimarco B, Iaccarino G, Ciccarelli M (2015) Integrating GRK2 and NFκB in the pathophysiology of cardiac hypertrophy. J Cardiovasc Transl Res 8:493–502. https://doi.org/10.1007/s12265-015-9646-0
Takahashi M, Tanonaka K, Yoshida H, Koshimizu M, Daicho T, Oikawa R, Takeo S (2006) Possible involvement of calpain activation in pathogenesis of chronic heart failure after acute myocardial infarction. J Cardiovasc Pharmacol 47:413–421. https://doi.org/10.1097/01.fjc.0000210074.56614.3b
Taneike M, Mizote I, Morita T, Watanabe T, Hikoso S, Yamaguchi O, Takeda T, Oka T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Takeda J, Mochizuki N, Komuro I, Otsu K (2011) Calpain protects the heart from hemodynamic stress. J Biol Chem 286:32170–32177. https://doi.org/10.1074/jbc.M111.248088
Toth A, Nickson P, Qin LL, Erhardt P (2006) Differential regulation of cardiomyocyte survival and hypertrophy by MDM2, an E3 ubiquitin ligase. J Biol Chem 281:3679–3689. https://doi.org/10.1074/jbc.M509630200
Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ (1993) Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation 87:454–463. https://doi.org/10.1161/01.CIR.87.2.454
Wan F, Letavernier E, Le Saux CJ, Houssaini A, Abid S, Czibik G, Sawaki D, Marcos E, Dubois-Rande JL, Baud L, Adnot S, Derumeaux G, Gellen B (2015) Calpastatin overexpression impairs postinfarct scar healing in mice by compromising reparative immune cell recruitment and activation. Am J Physiol Heart Circ Physiol 309:H1883–H1893. https://doi.org/10.1152/ajpheart.00594.2015
Wang Y, Chen B, Huang CK, Guo A, Wu J, Zhang X, Chen R, Chen C, Kutschke W, Weiss RM, Boudreau RL, Margulies KB, Hong J, Song LS (2018) Targeting calpain for heart failure therapy: implications from multiple murine models. JACC Basic Transl Sci 3:503–517. https://doi.org/10.1016/j.jacbts.2018.05.004
Yang D, Ma S, Tan Y, Li D, Tang B, Zhang X, Sun M, Yang Y (2010) Increased expression of calpain and elevated activity of calcineurin in the myocardium of patients with congestive heart failure. Int J Mol Med 26:159–164. https://doi.org/10.3892/ijmm_00000448
Ye T, Wang Q, Zhang Y, Song X, Yang D, Li D, Li D, Su L, Yang Y, Ma S (2015) Over-expression of calpastatin inhibits calpain activation and attenuates post-infarction myocardial remodeling. PLoS One 10:e0120178. https://doi.org/10.1371/journal.pone.0120178
Zha Z, Han X, Smith MD, Liu Y, Giguere PM, Kopanja D, Raychaudhuri P, Siderovski DP, Guan KL, Lei QY, Xiong Y (2015) A non-canonical function of Gbeta as a subunit of E3 ligase in targeting GRK2 ubiquitylation. Mol Cell 58:794–803. https://doi.org/10.1016/j.molcel.2015.04.017
Funding
This study was supported by Instituto de Salud Carlos III, Spain [PI-16/00232; RETICS-RIC-RD12/0042/0021 to D.G.D., co-funded with European Regional Development Fund-FEDER contribution], by Ministerio de Economía; Industria y Competitividad (MINECO) of Spain [SAF2017-84125-R to F.M.]; by CIBERCV-Instituto de Salud Carlos III, Spain [CB16/11/00479 to D.G.D. and CB16/11/00278 to F.M, co-funded with European Regional Development Fund-FEDER contribution], and Programa de Actividades en Biomedicina de la Comunidad de Madrid-B2017/BMD-3671-INFLAMUNE to F.M. We also acknowledge institutional support to the CBMSO from Fundación Ramón Areces. D.A. is a recipient of a VHIR predoctoral grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Aluja, D., Inserte, J., Penela, P. et al. Calpains mediate isoproterenol-induced hypertrophy through modulation of GRK2. Basic Res Cardiol 114, 21 (2019). https://doi.org/10.1007/s00395-019-0730-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-019-0730-5