Abstract
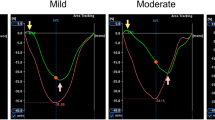
The reversibility of viable dysfunctional myocardium after revascularization is variable and the reasons for this are unknown. Using 2D-DIGE, we tested the hypothesis that this could reflect the extent of molecular remodeling of myocardial tissue in the absence of infarction. Swine with a progressive left anterior descending (LAD) stenosis were studied 2 months (n = 18) or 3 months (n = 22) post-instrumentation. Coronary flow reserve (vasodilated/rest) was severely reduced at 2 months (LAD 2.6 ± 0.4 versus 5.1 ± 0.4 in normal, p < 0.05) and became critically impaired after 3 months (LAD 1.1 ± 0.2, p < 0.05 vs. 2 months). Despite progression in stenosis severity, reductions in wall thickening at 2 months (LAD 37 ± 4 % vs. remote 86 ± 9 %, p < 0.05) were unchanged at 3 months (LAD 32 ± 3 %, p = ns). Contractile dysfunction was primarily related to reductions (LAD/normal) in contractile proteins which were not affected by stenosis severity (e.g., troponin T, 2 months 0.82 ± 0.03 vs. 0.74 ± 0.03 at 3 months, p-ns). In contrast, mitochondrial function and proteins were normal at 2 months but declined with progression to a critical stenosis (state 3 respiration at 3 months 145 ± 13 vs. 216 ± 5 ng-atoms O2 mg−1 min−1 at 2 months, p < 0.05). In a similar fashion, increases in stress (e.g., αB-crystalline 2.13 ± 0.2 vs. 1.17 ± 0.13 at 2 months, p < 0.05) and cytoskeletal proteins (e.g., desmin 1.63 ± 0.12 vs. 1.24 ± 0.10 at 2 months, p < 0.05) only developed with more advanced remodeling from a critical stenosis. We conclude that similar degrees of chronic contractile dysfunction can have diverse intrinsic molecular adaptations to ischemia. This spectrum of adaptations may underlie variability in the time course and extent of reversibility in viable chronically dysfunctional myocardium after revascularization.








Similar content being viewed by others
Abbreviations
- ADP:
-
Adenosine diphosphate
- ATP:
-
Adenosine triphosphate
- EGTA:
-
Ethylene glycol tetraacetic acid
- HEPES:
-
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HSP:
-
Heat shock protein
- IM:
-
Intramuscular
- IV:
-
Intravenous
- KCl:
-
Potassium chloride
- MOPS:
-
3-(N-morpholino)propanesulfonic acid
References
Angelini A, Maiolino G, La Canna G, Ceconi C, Calabrese F, Pettenazzo E, Valente M, Alfieri O, Thiene G, Ferrari R (2007) Relevance of apoptosis in influencing recovery of hibernating myocardium. Eur J Heart Fail 9:377–383. doi:10.1016/j.ejheart.2006.09.012
Arrell DK, Elliott ST, Kane LA, Guo Y, Ko YH, Pedersen PL, Robinson J, Murata M, Murphy AM, Marban E, Van Eyk JE (2006) Proteomic analysis of pharmacological preconditioning: novel protein targets converge to mitochondrial metabolism pathways. Circ Res 99:706–714. doi:10.1161/01.RES.0000243995.74395.f8
Baker CS, Dutka DP, Pagano D, Rimoldi O, Pitt M, Hall RJ, Polak JM, Bonser RS, Camici PG (2002) Immunocytochemical evidence for inducible nitric oxide synthase and cyclooxygenase-2 expression with nitrotyrosine formation in human hibernating myocardium. Basic Res Cardiol 97:409–415
Borgers M, Ausma J (1995) Structural aspects of the chronic hibernating myocardium in man. Basic Res Cardiol 90:44–46
Canty JM Jr, Fallavollita JA (2005) Hibernating myocardium. J Nucl Cardiol 12:104–119. doi:10.1016/j.nuclcard.2004.11.003
Canty JM Jr, Suzuki G (2012) Myocardial perfusion and contraction in acute ischemia and chronic ischemic heart disease. J Mol Cell Cardiol 52:822–831. doi:10.1016/j.yjmcc.2011.08.019
Duan X, Young RF, Straubinger RM, Page BJ, Cao J, Wang H, Yu Y, Canty JM Jr, Qu J (2009) A straightforward and highly efficient precipitation/on-pellet digestion procedure coupled to a long gradient nano-LC separation and Oprbitrap mass spectrometry for label-free expression profiling of the swine heart mitochondrial proteome. J Proteome Res 8:2838–2850. doi:10.1021/pr900001t
Fallavollita JA, Canty JM Jr (1999) Differential 18F-2-deoxyglucose uptake in viable dysfunctional myocardium with normal resting perfusion: evidence for chronic stunning in pigs. Circulation 99:2798–2805. doi:10.1161/01.CIR.99.21.2798
Fallavollita JA, Jacob SC, Young RF, Canty JM Jr (1999) Regional alterations in SR Ca2+ ATPase, phospholamban, and HSP-70 expression in chronic hibernating myocardium. Am J Physiol Heart Circ Physiol 277:H1418–H1428
Fallavollita JA (2000) Spatial heterogeneity in fasting and insulin-stimulated (18)F-2-deoxyglucose uptake in pigs with hibernating myocardium. Circulation 102:908–914. doi:10.1161/01.CIR.102.8.908
Fallavollita JA, Lim H, Canty JM Jr (2001) Myocyte apoptosis and reduced SR gene expression precede the transition from chronically stunned to hibernating myocardium. J Mol Cell Cardiol 33:1937–1944. doi:10.1006/jmcc.2001.1457
Fallavollita JA, Logue M, Canty JM Jr (2001) Stability of hibernating myocardium in pigs with a chronic left anterior descending coronary artery stenosis: absence of progressive fibrosis in the setting of stable reductions in flow, function and coronary flow reserve. J Am Coll Cardiol 37:1989–1995. doi:10.1016/S0735-1097(01)01250-5
Fallavollita JA, Malm BJ, Canty JM Jr (2003) Hibernating myocardium retains metabolic and contractile reserve despite regional reductions in flow, function, and oxygen consumption at rest. Circ Res 92:48–55. doi:10.1161/01.RES.0000049104.57549.03
Fallavollita JA, Perry BJ, Canty JM Jr (1997) 18F-2-deoxyglucose deposition and regional flow in pigs with chronically dysfunctional myocardium: evidence for transmural variations in chronic hibernating myocardium. Circulation 95:1900–1909. doi:10.1161/01.CIR.95.7.1900
Heusch G (1998) Hibernating myocardium. Physiol Rev 78:1055–1085
Heusch G, Schulz R, Rahimtoola SH (2005) Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol 288:H984–H999
Hoppel C, DiMarco JP, Tandler B (1979) Riboflavin and rat hepatic cell structure and function. Mitochondrial oxidative metabolism in deficiency states. J Biol Chem 254:4164–4170
Hu Q, Suzuki G, Young RF, Page BJ, Fallavollita JA, Canty JM Jr (2009) Reductions in mitochondrial O(2) consumption and preservation of high-energy phosphate levels after simulated ischemia in chronic hibernating myocardium. Am J Physiol Heart Circ Physiol 297:H223–H232. doi:10.1152/ajpheart.00992.2008
Kassiotis C, Rajabi M, Taegtmeyer H (2008) Metabolic reserve of the heart: the forgotten link between contraction and coronary flow. Prog Cardiovasc Dis 51:74–88. doi:10.1016/j.pcad.2007.11.005
Kelly RF, Cabrera JA, Ziemba EA, Crampton M, Anderson LB, McFalls EO, Ward HB (2011) Continued depression of maximal oxygen consumption and mitochondrial proteomic expression despite successful coronary artery bypass grafting in a swine model of hibernation. J Thorac Cardiovasc Surg 141:261–268. doi:10.1016/j.jtcvs.2010.08.061
Kim SJ, Peppas A, Hong SK, Yang G, Huang Y, Diaz G, Sadoshima J, Vatner DE, Vatner SF (2003) Persistent stunning induces myocardial hibernation and protection: flow/function and metabolic mechanisms. Circ Res 92:1233–1239. doi:10.1161/01.RES.0000076892.18394.B6
Kudej RK, White LT, Kudej AB, Vatner SF, Lewandowski ED (2002) Brief increase in carbohydrate oxidation after reperfusion reverses myocardial stunning in conscious pigs. Circulation 106:2836–2841. doi:10.1161/01.CIR.0000039326.87475.98
Lim H, Fallavollita JA, Hard R, Kerr CW, Canty JM Jr (1999) Profound apoptosis-mediated regional myocyte loss and compensatory hypertrophy in pigs with hibernating myocardium. Circulation 100:2380–2386. doi:10.1161/01.CIR.100.23.2380
Luss H, Boknik P, Heusch G, Muller FU, Neumann J, Schmitz W, Schulz R (1998) Expression of calcium regulatory proteins in short-term hibernation and stunning in the in situ porcine heart. Cardiovasc Res 37:606–617
Malm BJ, Suzuki G, Canty JM Jr, Fallavollita JA (2002) Variability of contractile reserve in hibernating myocardium: dependence on the method of stimulation. Cardiovasc Res 56:422–433. doi:10.1016/S0008-6363(02)00599-0
McFalls EO, Sluiter W, Schoonderwoerd K, Manintveld OC, Lamers JM, Bezstarosti K, van Beusekom HM, Sikora J, Ward HB, Merkus D, Duncker DJ (2006) Mitochondrial adaptations within chronically ischemic swine myocardium. J Mol Cell Cardiol 41:980–988. doi:10.1016/j.yjmcc.2006.07.008
Page B, Young R, Iyer V, Suzuki G, Lis M, Korotchkina K, Patel M, Blumenthal K, Fallavollita JA, Canty JM Jr (2008) Persistent regional downregulation in mitochondrial enzymes and upregulation of stress proteins in swine with chronic hibernating myocardium. Circ Res 102:103–112. doi:10.1161/CIRCRESAHA.107.155895
Rahimtoola SH (1989) The hibernating myocardium. Am Heart J 117:211–221
Rahimtoola SH (1985) A perspective on the three large multicenter randomized clinical trials of coronary bypass surgery for chronic stable angina. Circulation 72(suppl V):V123–V135
Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H (2001) Metabolic gene expression in fetal and failing human heart. Circulation 104:2923–2931. doi:10.1161/hc4901.100526
Schinkel AF, Bax JJ, Delgado V, Poldermans D, Rahimtoola SH (2010) Clinical relevance of hibernating myocardium in ischemic left ventricular dysfunction. Am J Med 123:978–986. doi:10.1016/j.amjmed.2010.03.025
Thijssen VL, Borgers M, Lenders M-H, Ramaekers FC, Suzuki G, Palka B, Fallavollita JA, Thomas SA, Canty JM Jr (2004) Temporal and spatial variations in structural protein expression during the progression from stunned to hibernating myocardium. Circulation 110:3313–3321. doi:10.1161/01.CIR.0000147826.13480.99
Thomas SA, Fallavollita JA, Borgers M, Canty JM Jr (2002) Dissociation of regional adaptations to ischemia and global myolysis in an accelerated swine model of chronic hibernating myocardium. Circ Res 91:970–977. doi:10.1161/01.RES.0000040396.79379.77
Thomas SA, Fallavollita JA, Lee TC, Feng J, Canty JM Jr (1999) Absence of troponin I degradation or altered sarcoplasmic reticulum uptake protein expression after reversible ischemia in swine. Circ Res 85:446–456. doi:10.1161/01.RES.85.5.446
Vanoverschelde JL, Melin JA (2001) The pathophysiology of myocardial hibernation: current controversies and future directions. Prog Cardiovasc Dis 43:387–398. doi:10.1053/pcad.2001.20655
White MY, Edwards AVG, Cordwell SJ, Van Eyk JE (2008) Mitochondria: a mirror into cellular dysfunction in heart disease. Proteomics Clin Appl 2:845–861. doi:10.1002/prca.200780135
Zhang J, Liem DA, Mueller M, Wang Y, Zong C, Deng N, Vondriska TM, Korge P, Drews O, Maclellan WR, Honda H, Weiss JN, Apweiler R, Ping P (2008) Altered proteome biology of cardiac mitochondria under stress conditions. J Proteome Res 7:2204–2214. doi:10.1021/pr070371f
Acknowledgments
We would like to thank Anne Coe, Deana Gretka, Elaine Granica and Beth Palka for technical assistance.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. Supported by HL-55324, HL61610 and the Albert and Elizabeth Rekate Fund.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Page, B.J., Young, R.F., Suzuki, G. et al. The physiological significance of a coronary stenosis differentially affects contractility and mitochondrial function in viable chronically dysfunctional myocardium. Basic Res Cardiol 108, 354 (2013). https://doi.org/10.1007/s00395-013-0354-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-013-0354-0