Abstract
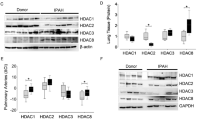
Nox4 is a member of the NADPH oxidase family, which represents a major source of reactive oxygen species (ROS) in the vascular wall. Nox4-mediated ROS production mainly depends on the expression levels of the enzyme. The present study was aimed to investigate the mechanisms of Nox4 transcription regulation by histone deacetylases (HDAC). In human umbilical vein endothelial cells (HUVEC) and HUVEC-derived EA.hy 926 cells, treatment with the pan-HDAC inhibitor scriptaid led to a marked decrease in Nox4 mRNA expression. A similar down-regulation of Nox4 mRNA expression was observed by siRNA-mediated knockdown of HDAC3. HDAC inhibition in endothelial cells was associated with enhanced histone acetylation, increased chromatin accessibility in the human Nox4 promoter region, with no significant changes in DNA methylation. In addition, we provided evidence that c-Jun played an important role in controlling Nox4 transcription. Knockdown of c-Jun with siRNA led to a down-regulation of Nox4 mRNA expression. In response to scriptaid treatment, the binding of c-Jun to the Nox4 promoter region was reduced despite the open chromatin structure. In parallel, the binding of RNA polymerase IIa to the Nox4 promoter was significantly inhibited as well, which may explain the reduction in Nox4 transcription. In conclusion, HDAC inhibition decreases Nox4 transcription in human endothelial cells by preventing the binding of transcription factor(s) and polymerase(s) to the Nox4 promoter, most likely because of a hyperacetylation-mediated steric inhibition.






Similar content being viewed by others
References
Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M (2004) Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109:227–233. doi:10.1161/01.CIR.0000105680.92873.70
Alam S, Li H, Margariti A, Martin D, Zampetaki A, Habi O, Cockerill G, Hu Y, Xu Q, Zeng L (2011) Galectin-9 protein expression in endothelial cells is positively regulated by histone deacetylase 3. J Biol Chem 286:44211–44217. doi:10.1074/jbc.M111.242289
Ballestar E (2011) An introduction to epigenetics. Adv Exp Med Biol 711:1–11
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313. doi:10.1152/physrev.00044.2005
Blackwell L, Norris J, Suto CM, Janzen WP (2008) The use of diversity profiling to characterize chemical modulators of the histone deacetylases. Life Sci 82:1050–1058. doi:10.1016/j.lfs.2008.03.004
Brandes RP, Kreuzer J (2005) Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65:16–27. doi:10.1016/j.cardiores.2004.08.007
Diebold I, Petry A, Hess J, Gorlach A (2010) The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 21:2087–2096. doi:10.1091/mbc.E09-12-1003
Dokmanovic M, Clarke C, Marks PA (2007) Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 5:981–989. doi:10.1158/1541-7786.MCR-07-0324
Drummond GR, Selemidis S, Griendling KK, Sobey CG (2011) Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10:453–471. doi:10.1038/nrd3403
Edgell CJ, McDonald CC, Graham JB (1983) Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 80:3734–3737
Eferl R, Wagner EF (2003) AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3:859–868. doi:10.1038/nrc1209
Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR (2005) The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res 65:495–504. doi:10.1016/j.cardiores.2004.10.026
Farre D, Roset R, Huerta M, Adsuara JE, Rosello L, Alba MM, Messeguer X (2003) Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res 31:3651–3653
Forstermann U (2008) Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med 5:338–349. doi:10.1038/ncpcardio1211
Fu Y, Zhang Y, Wang Z, Wang L, Wei X, Zhang B, Wen Z, Fang H, Pang Q, Yi F (2010) Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am J Nephrol 32:581–589. doi:10.1159/000322105
Fuks F, Burgers WA, Godin N, Kasai M, Kouzarides T (2001) Dnmt3a binds deacetylases and is recruited by a sequence-specific repressor to silence transcription. EMBO J 20:2536–2544. doi:10.1093/emboj/20.10.2536
Goettsch C, Goettsch W, Brux M, Haschke C, Brunssen C, Muller G, Bornstein SR, Duerrschmidt N, Wagner AH, Morawietz H (2011) Arterial flow reduces oxidative stress via an antioxidant response element and Oct-1 binding site within the NADPH oxidase 4 promoter in endothelial cells. Basic Res Cardiol 106:551–561. doi:10.1007/s00395-011-0170-3
Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ (2008) Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 22:3549–3560. doi:10.1096/fj.08-108548
Griendling KK (2004) Novel NAD(P)H oxidases in the cardiovascular system. Heart 90:491–493
Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, Channon KM (2006) Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 26:333–339. doi:10.1161/01.ATV.0000196651.64776.51
Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H (2003) Role of oxidative stress in atherosclerosis. Am J Cardiol 91:7A–11A
Hwang J, Kleinhenz DJ, Lassegue B, Griendling KK, Dikalov S, Hart CM (2005) Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol 288:C899–C905. doi:10.1152/ajpcell.00474.2004
Im YB, Jee MK, Jung JS, Choi JI, Jang JH, Kang SK (2012) miR23b ameliorates neuropathic pain in spinal cord by silencing NADPH oxidase 4. Antioxid Redox Signal 16:1046–1060. doi:10.1089/ars.2011.4224
Jung SB, Kim CS, Naqvi A, Yamamori T, Mattagajasingh I, Hoffman TA, Cole MP, Kumar A, Dericco JS, Jeon BH, Irani K (2010) Histone deacetylase 3 antagonizes aspirin-stimulated endothelial nitric oxide production by reversing aspirin-induced lysine acetylation of endothelial nitric oxide synthase. Circ Res 107:877–887. doi:10.1161/CIRCRESAHA.110.222968
Katsuyama M, Hirai H, Iwata K, Ibi M, Matsuno K, Matsumoto M, Yabe-Nishimura C (2011) Sp3 transcription factor is crucial for transcriptional activation of the human NOX4 gene. FEBS J 278:964–972. doi:10.1111/j.1742-4658.2011.08018.x
Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D (2002) The human genome browser at UCSC. Genome Res 12:996–1006. doi:10.1101/gr.229102 Article published online before print in May 2002
Klose RJ, Bird AP (2006) Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci 31:89–97. doi:10.1016/j.tibs.2005.12.008
Kroller-Schon S, Schulz E, Wenzel P, Kleschyov AL, Hortmann M, Torzewski M, Oelze M, Renne T, Daiber A, Munzel T (2011) Differential effects of heart rate reduction with ivabradine in two models of endothelial dysfunction and oxidative stress. Basic Res Cardiol 106:1147–1158. doi:10.1007/s00395-011-0227-3
LaBonte MJ, Wilson PM, Fazzone W, Groshen S, Lenz HJ, Ladner RD (2009) DNA microarray profiling of genes differentially regulated by the histone deacetylase inhibitors vorinostat and LBH589 in colon cancer cell lines. BMC Med Genomics 2:67. doi:10.1186/1755-8794-2-67
Lassegue B, Clempus RE (2003) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285:R277–R297. doi:10.1152/ajpregu.00758.2002
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Manea A, Tanase LI, Raicu M, Simionescu M (2010) Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. Arterioscler Thromb Vasc Biol 30:105–112. doi:10.1161/ATVBAHA.109.193896
Manea A, Tanase LI, Raicu M, Simionescu M (2010) Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem Biophys Res Commun 396:901–907. doi:10.1016/j.bbrc.2010.05.019
Matouk CC, Marsden PA (2008) Epigenetic regulation of vascular endothelial gene expression. Circ Res 102:873–887. doi:10.1161/CIRCRESAHA.107.171025
Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM (2002) PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 18:333–334
Miyano K, Ueno N, Takeya R, Sumimoto H (2006) Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J Biol Chem 281:21857–21868. doi:10.1074/jbc.M513665200
Morawietz H (2011) Endothelial NADPH oxidases: friends or foes? Basic Res Cardiol 106:521–525. doi:10.1007/s00395-011-0188-6
Murdoch CE, Alom-Ruiz SP, Wang MS, Zhang M, Walker S, Yu B, Brewer A, Shah AM (2011) Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol 106:527–538. doi:10.1007/s00395-011-0179-7
Park Y, Yang JY, Zhang HR, Chen XN, Zhang CH (2011) Effect of PAR2 in regulating TNF-alpha and NAD(P)H oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol 106:111–123. doi:10.1007/s00395-010-0129-9
Pendyala S, Moitra J, Kalari S, Kleeberger SR, Zhao Y, Reddy SP, Garcia JG, Natarajan V (2011) Nrf2 regulates hyperoxia-induced Nox4 expression in human lung endothelium: identification of functional antioxidant response elements on the Nox4 promoter. Free Radic Biol Med 50:1749–1759. doi:10.1016/j.freeradbiomed.2011.03.022
Rossig L, Li H, Fisslthaler B, Urbich C, Fleming I, Forstermann U, Zeiher AM, Dimmeler S (2002) Inhibitors of histone deacetylation downregulate the expression of endothelial nitric oxide synthase and compromise endothelial cell function in vasorelaxation and angiogenesis. Circ Res 91:837–844
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Ruf N, Bahring S, Galetzka D, Pliushch G, Luft FC, Nurnberg P, Haaf T, Kelsey G, Zechner U (2007) Sequence-based bioinformatic prediction and QUASEP identify genomic imprinting of the KCNK9 potassium channel gene in mouse and human. Hum Mol Genet 16:2591–2599. doi:10.1093/hmg/ddm216
Sirker A, Zhang M, Shah AM (2011) NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol 106:735–747. doi:10.1007/s00395-011-0190-z
Smith CL (2008) A shifting paradigm: histone deacetylases and transcriptional activation. BioEssays 30:15–24. doi:10.1002/bies.20687
Strahl BD, Allis CD (2000) The language of covalent histone modifications. Nature 403:41–45. doi:10.1038/47412
Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR (2006) Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290:L661–L673. doi:10.1152/ajplung.00269.2005
Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP (2011) The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286:13304–13313. doi:10.1074/jbc.M110.192138
Vasa-Nicotera M, Chen H, Tucci P, Yang AL, Saintigny G, Menghini R, Mahe C, Agostini M, Knight RA, Melino G, Federici M (2011) miR-146a is modulated in human endothelial cell with aging. Atherosclerosis 217:326–330. doi:10.1016/j.atherosclerosis.2011.03.034
Xia N, Daiber A, Habermeier A, Closs EI, Thum T, Spanier G, Lu Q, Oelze M, Torzewski M, Lackner KJ, Munzel T, Forstermann U, Li H (2010) Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J Pharmacol Exp Ther 335:149–154. doi:10.1124/jpet.110.168724
Xu H, Czerwinski P, Hortmann M, Sohn HY, Forstermann U, Li H (2008) Protein kinase C alpha promotes angiogenic activity of human endothelial cells via induction of vascular endothelial growth factor. Cardiovasc Res 78:349–355. doi:10.1093/cvr/cvm085
Xu H, Goettsch C, Xia N, Horke S, Morawietz H, Forstermann U, Li H (2008) Differential roles of PKCalpha and PKCepsilon in controlling the gene expression of Nox4 in human endothelial cells. Free Radic Biol Med 44:1656–1667. doi:10.1016/j.freeradbiomed.2008.01.023
Zampetaki A, Zeng L, Margariti A, Xiao Q, Li H, Zhang Z, Pepe AE, Wang G, Habi O, deFalco E, Cockerill G, Mason JC, Hu Y, Xu Q (2010) Histone deacetylase 3 is critical in endothelial survival and atherosclerosis development in response to disturbed flow. Circulation 121:132–142. doi:10.1161/CIRCULATIONAHA.109.890491
Zhang L, Sheppard OR, Shah AM, Brewer AC (2008) Positive regulation of the NADPH oxidase NOX4 promoter in vascular smooth muscle cells by E2F. Free Radic Biol Med 45:679–685. doi:10.1016/j.freeradbiomed.2008.05.019
Zhang YS, He L, Liu B, Li NS, Luo XJ, Hu CP, Ma QL, Zhang GG, Li YJ, Peng J (2012) A novel pathway of NADPH oxidase/vascular peroxidase 1 in mediating oxidative injury following ischemia-reperfusion. Basic Res Cardiol 107:266. doi:10.1007/s00395-012-0266-4
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft [DFG, grant LI-1042/1-1], by the Federal Ministry of Education and Research (BMBF 01EO1003), and by a grant from the University Medical Center (Schwerpunkt Vaskuläre Prävention). D. Langer was supported by a PhD-scholarship of the Studienstiftung des deutschen Volkes. We thank Gisela Reifenberg for excellent technical assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Siuda, D., Zechner, U., El Hajj, N. et al. Transcriptional regulation of Nox4 by histone deacetylases in human endothelial cells. Basic Res Cardiol 107, 283 (2012). https://doi.org/10.1007/s00395-012-0283-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-012-0283-3