Abstract
Regular exercise can lead to electrical remodelling of the heart. The cellular mechanisms associated with these changes are not well understood, and are difficult to study in human tissue but are important given that exercise is recommended to the general population. We have investigated the role played by the transient outward K+ current (I to) in the changes in electrical activity seen in response to voluntary exercise training in rats. Female rats undertook 6 weeks of voluntary wheel running exercise (TRN) or were sedentary controls (SED). Monophasic action potentials (MAPs) were recorded from the surface of whole hearts. Whole cell patch clamp recordings of I to; mRNA and protein levels of selected targets in sub-epicardial (EPI) and sub-endocardial myocardium of SED and TRN hearts were compared. In TRN rats, heart weight:body weight was significantly increased and epicardial MAPs significantly prolonged. I to density was reduced in TRN EPI myocytes, such that the transmural gradient of I to was significantly reduced (P < 0.05). Computer modelling of these changes in I to predicted the observed changes in action potential profile. However, transmural gradients in mRNA and protein expression for Kv4.2 or mRNA levels of the Kv4.2 regulators; KChIP2 and Irx-5 were not significantly altered by voluntary exercise. We conclude that voluntary exercise electrical remodelling is caused, at least in part, by a decrease in EPI I to, possibly because of fewer functional channels in the membrane, which results in a fall in the transmural action potential duration gradient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cardiovascular benefits of regular physical activity in humans are acknowledged [11] and a variety of animal models, employing either voluntary or enforced methods of exercise training, have been used to investigate the cellular mechanisms associated with the cardiovascular adaptations to exercise [8, 18, 25].
Exercise training can alter the electrical activity of the heart and understanding this electrical remodelling is important. In humans, the electrocardiogram (ECG) can undergo modifications in response to regular exercise that mimic changes associated with pro-arrhythmic states such as hypertrophic cardiomyopathy, e.g. [15, 21, 31, 32] although, the causes and outcomes of physiological and pathological remodelling are usually different, e.g. [10].
The cellular mechanisms that cause these changes in ECG configuration, in response to exercise, are unknown and are difficult to study directly in human tissue. A model such as the voluntary running rat that promotes mild exercise as recommended to the general population is therefore a valuable research tool.
We have previously reported a prolongation of the action potential duration (APD) in single myocytes isolated from the sub-epicardial (EPI) but not the sub-endocardial (ENDO) region of the left ventricle of female rats that had undergone a period of voluntary exercise training [27]. In many mammalian species, a transmural gradient in the activity of the transient outward repolarising current (I to), carried by several Kv channels, underpins regional differences in APD [1, 3, 29]. In this study, we wished to test the hypothesis that the transmural gradient of I to is modified by voluntary exercise training and that this may contribute to changes in APD.
Methods
All animal experimentation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and UK Home Office Animals (Scientific Procedures) Act 1986.
Exercise training model
Sixty-nine female Sprague-Dawley rats were weight and aged matched and assigned randomly to either a sedentary (SED) or exercise trained (TRN) group. Female rats were used as they have been reported to be more likely to run spontaneously than male rats, e.g. [33]. TRN rats had free access to vertical running wheels over a 6-week period as previously described [27, 40], SED animals were housed as standard laboratory rats with no access to wheels. Individual running distances were recorded daily and animals weighed weekly.
Monophasic action potentials in the whole heart
At the end of the training period, rats were weighed and killed humanely. Hearts were removed and flushed with a bicarbonate-buffered solution of the following composition (mM): 118.5 NaCl, 14.5 Na HCO3, 4.2 KCl, 1.2 KH2PO4, 1.2 MgSO4 · 7H2O, 11.1 Glucose, 1 CaCl2, pH 7.4, blotted dry and weighed before Langendorff perfusion at 0.11 ml s−1 g−1 heart weight with the above solution at 37°C. Hearts were paced at a stimulation frequency of 5 Hz via platinum electrodes close to the junction of the right atria and right ventricle. A Franz-type monophasic action potential (MAP) electrode was used to record MAPs from the EPI surface of the left ventricle in the mid-line region within 15–20 min of excision of the heart. MAP criteria were as previously described [12].
Cardiac myocyte isolation
Hearts were excised and single cardiac myocytes were isolated using a collagenase-type XIV-protease dispersion technique [22]. At the end of the initial enzyme perfusion period, the ventricles were separated and weighed. Tissue was dissected from the left ventricular EPI and ENDO layer, leaving a distinct and dividing mid-myocardial layer, and single EPI and ENDO myocytes were then isolated by mechanical dispersion. Cells were stored at 21°C in a modified HEPES-Tyrode solution which contained (mM): 130 NaCl, 5.4 KCl, 1.4 MgCl2, 0.4 NaH2PO4, 5 HEPES, 10 glucose, 10 creatine, 20 taurine, 0.75 CaCl2 adjusted to pH 7.4 with NaOH and used within 8 h.
Electrophysiology
Myocytes were superfused with a solution containing (mM): 150 NaCl, 5.4 KCl, 2 MgCl2, 10 glucose, 10 HEPES, 0.6 CaCl2 and 10 μM nifedipine, adjusted to pH 7.4 with NaOH. K+ currents were recorded at 37°C using the whole cell patch clamp technique with an Axopatch 1D amplifier (Axon Instruments). Micropipettes had resistances of 1.5–2.5 MΩ when filled with a solution containing (mM): 140 KCl, 4 MgCl2, 5 CaCl2, 10 EGTA, 10 HEPES, and 4 Na2ATP, adjusted to pH 7.3 with KOH. Myocytes were stimulated at 0.2 Hz. Cell capacitance was measured prior to compensation. Access resistance was below 7 MΩ and series resistance and capacitance were compensated. I to was measured in response to membrane depolarisations between −40 and +60 mV (see Fig. 2a) and was separated from steady state current (I ss) by pre-pulse voltage [16]. Current density was expressed as pA/pF for each cell. I to was also expressed as % conductance by dividing the current by the driving force for K+ (assuming an equilibrium potential of −85 mV for K+) and expressed as a % of conductance at +60 mV see [6].
Isolation of RNA and RT-PCR
Left ventricular free wall samples from ten TRN and ten SED rats were held flat between the broadened ends of a pair of tongues and snap frozen in liquid nitrogen. The thickness of the resulting slab was measured with cold calipers and EPI, and ENDO thirds harvested on a cryostat. Total RNA extraction was performed using a modified Qiagen mini-kit protocol for striated muscle as previously described [39, 40, 46]. Real-time RT-PCR was performed using either a Roche LightCycler (Roche Applied Systems, Indianapolis, IN, USA) with SYBRGreen detection or the ABI PRISM 7900HT sequence detection system (Applied Biosystems). For the LightCycler, specific primers were designed to amplify target genes (Kv1.4 and KChIP2) with the aid of the primer 3 program (see Table 1). For the ABI PRISM, pre-designed primers were used. Relative transcript expression was normalized to the housekeeper gene GAPDH.
Western blotting
Fifty microgram of total protein per lane from EPI and ENDO single myocytes from TRN and SED hearts were separated on 10% Tris–HCl gels, followed by semi-dry transfer of the proteins onto PVDF membranes and probing by antibodies. Primary antibodies were a rabbit polyclonal antibody raised against amino acids 23–43 of rat Kv4.2 (1:1,000, Abcam, UK, #ab16719, overnight at 4°C), and anti-GAPDH antibody produced in rabbit raised against amino acids 214–333 of mouse GAPDH (0.1 μg/ml, Sigma USA, G9545, overnight at 4°C). Anti-GAPDH was used as a loading control for protein normalization. Signals were visualized with anti-rabbit IgG horseradish peroxidase conjugated secondary antibodies (1:10,000, #1858415, Pierce USA for anti-Kv4.2 and 1:5,000, #111-035-144, Jackson Immuno Research Laboratories Inc., USA for anti-GAPDH) and using an ECL detection system (Supersignal West Dura Extended Duration Substrate, #34075, Pierce, USA for anti-Kv4.2 and Supersignal West Pico Chemiluminescent Substrate, #34080, Pierce, USA, for anti-GAPDH). Band density of Kv4.2 was determined using Scion Image for Windows (Scion Corporation, USA), and normalized to the band density of GAPDH.
Computer modelling
The rat ventricular cell model developed by Pandit et al. [28] was used for this study because it includes recent advances in rat electrophysiology including rat transmural APD gradients. To simulate the effects of exercise-induced changes in the I to and I ss current densities (as shown in Figs. 2, 3) the maximal channel conductance of I to and I ss in the EPI and ENDO cell models were modified accordingly. To evoke action potentials, a series of five supra-threshold stimuli, with an amplitude of 0.6 nA and duration of 5 ms was applied at a frequency of 5 Hz. The fourth action potential was used for analysis.
A multicellular model of a 1D transmural ventricular strand was constructed using the following equation:
where I tot is the total ionic current (normalized by cell capacitance) membrane and is calculated by the Pandit et al. [28] model. D is the diffusion parameter describing the intercellular electrical coupling via gap junctions [48]. In simulations, D was a constant value of 1.13 × 10−2 mm2 s−1 that gave a conduction velocity of a planar wave across the ventricular wall of 0.14 m s−1, close to the measured velocity in cultured rat ventricular tissue [23]. The 1D strand has a total length of 3 mm, of which 2 mm was designated ENDO and 1 mm EPI tissue. Due to the lack of detailed experimental data, the proportion of the two distinctive regions was chosen empirically to produce a positive T-wave in the computed pseudo-ECG under control (sedentary) conditions. The simulated strand employed a spatial resolution of 0.1 mm—close to the length of ventricular myocytes, which generated 20 nodes for the ENDO segment and ten nodes for the EPI segment. As the spatial resolution is close to the cell length of ventricular myocytes (80–150 μm), it is adequate for assumption of isopotentiality of a node of the model. Equation 1 was numerically solved by the explicit Euler method with a time step of 0.1 μs. The chosen space and time steps guarantee a stable solution of Eq. 1. The pseudo-ECG was computed following the method of Gima and Rudy [13].
Statistics
Data are expressed as mean ± SEM. Statistical analysis was performed (with Sigmastat 3.0, SPSS) by two-way analysis of variance (two-way ANOVA) to assess for the influence of exercise (TRN vs. SED), region (EPI vs. ENDO) and interaction of these factors [2]. When significant differences (P < 0.05) were established, appropriate pairwise comparisons between individual groups were made.
Results
Effect of exercise on whole heart size and electrophysiology
Daily running distances increased over the first 4 weeks of training from 4.0 ± 0.2 km/day in week 1, to 11.4 ± 0.7 km/day by week 4 (Fig. 1a). There was a decrease in mean body weight (from 222.7 ± 3.1 to 213.3 ± 3.0 g, P < 0.05) and an increase in mean heart weight (from 1.14 ± 0.03 to 1.24 ± 0.028 g, P < 0.05) of TRN animals compared to control SED, thus cardiac hypertrophy in the TRN group was confirmed by significant increases in heart and ventricular weight to body weight ratios (Fig. 1b, P < 0.001). The capacitance of cells used in the electrophysiological studies was greater in ENDO than EPI myocytes in both trained and sedentary rats. Voluntary exercise increased myocyte size, as indicated by cell capacitance, by 15% compared to the sedentary group (TRN EPI 129 ± 6 pF, n = 28, TRN ENDO 176 ± 17 pF *, n = 13, SED EPI 109 ± 8 pF, n = 23, SED ENDO 146 ± 5 pF, n = 14, * P < 0.05, two-way ANOVA, effect of exercise).
Voluntary running distances and indices of cardiac hypertrophy. a Mean daily voluntary running distances in 37 rats. b Heart weight to body weight (HW:BW SED n = 32, TRN n = 37), ventricular weight to body weight (VW:BW SED n = 22, TRN n = 29) and left ventricular weight to body weight (LVW:BW SED n = 16, TRN n = 21) ratios for TRN and SED rats. All three ratios were significantly greater in TRN compared to SED rats (*, unpaired t test, P < 0.001 HW:BW, P < 0.05 LVW:BW, Mann Whitney rank sum test, P = 0.002, VW:BW)
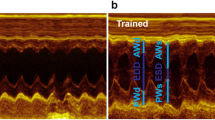
Significant lengthening of APD occurs in EPI, but not ENDO myocytes from TRN rats [27]. We therefore tested whether prolonged EPI APD was present (and therefore relevant) in the whole heart, by comparing MAPs from SED and TRN hearts. We observed a significant prolongation of EPI MAP duration in TRN hearts (Fig. 2).
Monophasic action potentials. a Example of monophasic action potentials recorded from the epicardial surface of a rat heart, stimulated at 5 Hz. b Monophasic action potential duration at 90% repolarisation was significantly greater in TRN compared to SED hearts (*P < 0.05, unpaired t test, n = 12 TRN, 10 SED hearts)
Transient outward (I to) and steady state (I ss) currents
I to and I ss were measured in single myocytes under whole cell voltage clamp conditions (Fig. 3a). Mean current–voltage relationships from SED animals showed significantly greater density of I to in EPI myocytes than ENDO myocytes, as predicted (Fig. 3b). We also observed a significantly greater density of I to in EPI myocytes than ENDO myocytes from TRN animals (Fig. 3c). However, the difference between the EPI and ENDO current–voltage relationships appeared smaller in TRN (Fig. 3c) than SED (Fig. 3b) myocytes, and two way ANOVA revealed that in addition to the regional differences in I to current, there was a significantly smaller I to in TRN EPI myocytes compared to TRN SED myocytes.
Outward K+ currents measured by whole cell voltage clamp. a (Inset) schematic diagram of the voltage clamp protocol used to isolate I to. Current recordings from a representative EPI myocyte elicited by stepping to potentials between −40 and +60 mV (for 300 ms) after a 2 s conditioning clamp step to −70 (a) or −20 mV (b). Records in panel (a) show activation of I to and I ss, panel (b) shows activation of I ss alone. I to is isolated by subtraction of traces in (a) and (b). I to in typical EPI (c ) and ENDO (d) myocytes of similar size, from a SED rat. Calibration bar represents 2 nA and 50 ms. b I to was significantly greater in EPI (n = 23) than ENDO (n = 14) myocytes from SED rats. c I to is also significantly greater in EPI (n = 28) than ENDO (n = 13) myocytes from TRN rats (*P < 0.05, two-way ANOVA). However, comparison of b and c suggests a smaller transmural gradient of I to
Figure 4 illustrates plots of the transmural gradient in I to (the difference between the mean EPI and mean ENDO current) at each voltage between 0 and +60 mV for SED and TRN animals. There was a significantly smaller transmural gradient of I to in myocytes from TRN animals than SED animals.
Transmural gradient of I to in myocytes from trained and sedentary rats. The difference between the mean EPI and mean ENDO I to currents (EPI-ENDO current) from Fig. 2 is plotted as a transmural gradient of I to for TRN and SED myocytes. The transmural gradient of I to is significantly reduced in TRN rats over the range of voltages shown (*P < 0.05, two-way ANOVA, n = 12 TRN, 10 SED hearts)
When I to was expressed as % conductance at +60 mV, there were no significant differences in I to activation curves between the four groups (data for relative conductance at +10 mV, close to half the conductance at +60 mV, is shown in Fig. 5a). The time constant of inactivating current, at +60 mV, of both EPI cell groups was significantly faster than both ENDO groups but the time constant of the two EPI and two ENDO groups were not significantly different from each other (Fig. 5b). The mean current–voltage relationships for I ss, were not different between the four groups of cells at +60 mV: ISS was 11.1 ± 1.3 pA/pF SED ENDO; 9.8 ± 0.7 pA/pF SED EPI; 10.4 ± 0.6 pA/pF TRN ENDO; 10.4 ± 0.8 pA/pF TRN EPI.
Activation and inactivation parameters for I to. a Activation of I to at +10 mV expressed as % conductance of that observed at +60 mV. There was no effect of region or training on the relative activation of I to (P > 0.05, two-way ANOVA). b Time constant of inactivation of I to at +60 mV. The inactivation of both EPI groups was significantly faster than both ENDO groups (*P < 0.05) but there was no effect of training on the inactivation of either EPI or ENDO myocytes (P > 0.05, two-way ANOVA, n = 13–23 myocytes)
Computer modelling of the effects of exercise on APD
To test whether this small but statistically significant effect of exercise training on EPI I to could explain the changes in APD we have previously observed, we used a computer model [28] that specifically addresses the differences in EPI and ENDO electrophysiology in the rat ventricle. Figure 6a models the statistically significant changes we observed in TRN EPI I to. It can be seen that this simulation generates data similar to that observed experimentally [27], i.e. a lengthening of APD in TRN EPI cells and a fall in the transmural gradient of APD. We therefore conclude that the changes in I to we report contribute to changes in APD. Additionally, the predicted change in the gradient was of sufficient magnitude to invert the T-wave of the simulated ECG, thus mimicking the changes in T-wave configuration observed in some humans in response to exercise training (see Fig. 4b, c and “Discussion”).
Simulated action potentials and pseudo-ECGs under trained and sedentary conditions. a Superimposed action potentials of ENDO and EPI cells under SED (S) and TRN (T) modelling changes in EPI I to. TRN prolonged APD and attenuated transmural APD dispersion. b Space–time plot of AP propagation across the 1D transmural ventricular strand. Membrane potential of cells along the strand is mapped into a colour spectrum ranging from dark blue for −80 mV to red for +40 mV (see colour key). Space runs vertically from the ENDO end at the bottom to the EPI end at the top. Time runs horizontally (left to right). c Simulated pseudo-ECG for SED and TRN conditions (see “Methods”) calculated from b. TRN conditions prolonged the QT interval and inverted the T-wave
Expression of mRNA for Kv channels and regulatory factors
I to is composed of at least two components; one is characterized by a relatively fast recovery from inactivation (I to,f) whose correlates are Kv4.2 and Kv4.3, the other has a slow recovery from inactivation (I to,s) and is carried by Kv1.4 [29]. Kv4.2 mRNA expression was greater in EPI than ENDO, but was not significantly altered by voluntary exercise. Kv4.3 and Kv1.4 expression were not influenced by region or exercise (Fig. 7a).
mRNA expression of selected Kv channels, accessory sub-units and modulating factors of Kv4.2. a Relative ratios of mRNA for EPI and ENDO samples from SED and TRN hearts. a Kv4.2 expression was dependent upon region, being significantly greater in EPI than ENDO (*P < 0.05 two-way ANOVA). Kv4.3 and Kv1.4 expression was not different between EPI and ENDO. Exercise training had no significant effect upon expression levels. b KChIP2 mRNA expression was significantly greater in EPI than ENDO, a significant but reversed transmural gradient was seen for Irx-5 (*P < 0.05, two-way ANOVA). There was no transmural gradient for calcineurin A beta. Voluntary exercise did not significantly alter the expression levels of any of the three targets (P > 0.05, two-way ANOVA). Expression of all mRNA levels is relative to GAPDH (n = 10 in all cases)
The activity of I to is not solely dependant upon the expression level of Kv transcripts. Kv4.2 subunits are influenced by accessory sub-units such a KChIP2 [14, 30, 35], the Iroquois homeobox transcription factor, Irx-5 [5, 34] and calcineurin A beta [36]. mRNA levels for KChIP2 and Irx-5 were region-dependent; expression of KChIP2 was greatest in EPI, Irx-5 was greatest in ENDO, though expression of calcineurin A beta was not influenced by region. However, the expression of these three regulatory factors was not affected by training (Fig. 7b).
Protein levels of Kv4.2
The expression of Kv4.2 protein in EPI and ENDO was assessed by Western blot analysis. This revealed two bands at ~79 and 74 kDa (see “Discussion”). Total Kv4.2 protein expression was calculated from the density of both immunoreactive bands and normalized to protein levels of GAPDH. We observed a higher level of protein in EPI than ENDO. However, although there was a tendency for the EPI:ENDO ratio to be lower in TRN hearts there was no significant differences between SED and TRN hearts (n = 6 SED, and 6 TRN) (Fig. 8).
Protein expression of Kv4.2. Upper panel, Kv4.2 protein in EPI and ENDO myocytes isolated from SED and TRN rats show greater Kv4.2 levels in EPI than ENDO. Lower panel gives EPI:ENDO for total Kv4.2 protein levels, normalized to the level of GAPDH for each sample. The ratios for SED and TRN were not significantly different from each other (P > 0.05, t test) (n = 6 in each group)
Discussion
Voluntary exercise model
When given voluntary use of an exercise wheel, female rats run considerable daily distances in frequent short bursts and achieve significant cardiac hypertrophy when compared to sedentary controls. Enforced exercise regimes can impose higher exercise intensities but additional stress responses may also be provoked [26, 47]. Enforced regimes may therefore be relevant to highly trained athletes while voluntary regimes are more suitable for the study of the response to mild exercise/enhanced activity levels. This is an important point given that regular mild exercise is recommended for the general population.
Electrophysiology
We have shown that voluntary exercise can induce a lengthening of the EPI MAPD in the intact heart, and consistent with this, a reduction in the density of EPI I to and a reduction in the transmural gradient of I to. These findings are the first to address the mechanisms of altered electrophysiology and to measure changes in ionic currents caused by voluntary (i.e. mild) exercise. The observation that MAP duration from the epicardial surface of trained hearts was lengthened shows that the electrical remodelling we have previously reported in single myocytes [27] persists in whole hearts, paced at a physiological frequency and is not cancelled by the electronic loading of cell neighbours in the multicellular environment [41] .
In hearts from SED rats the density of I to was greater in EPI myocytes compared to ENDO myocytes, as seen in previous studies in rats, e.g. [2, 3, 16]. The regional distribution of I to underlies the transmural gradient in APD in the rat ventricle [3, 29]. A novel observation of our study is the decrease in the transmural heterogeneity of I to in response to exercise training. Computer modelling predicts that this small but significant change in I to can largely explain the effects of voluntary exercise on the APD of single myocytes [27] and the MAP duration at the EPI surface of excised, intact hearts. Our modelling also predicts that in the absence of other influences, this change in I to would cause a depression of the ECG T-wave in a manner reported in some humans [15, 21, 31, 32]. It should be noted, however, that in vivo there are factors extrinsic to the myocardium such as the nervous and endocrine systems that may also modulate ECG parameters.
There is limited information on the effects of exercise training on ion channel activity [17, 24]. A training-induced increase in peak I to, and a decrease in I ss, has been shown [17]. However, in this study an enforced exercise regime was employed and the transmural origin of myocytes was not accounted for, making a direct comparison with this study difficult.
We observed a higher level of mRNA for Kv4.2, the dominant Kv channel sub-type responsible for I to in rat [29], in EPI than ENDO. Because this transmural distribution was not altered by exercise we investigated a potential influence of sub-units and other factors known to regulate Kv4.2 channel function. Several studies have shown that the differential expression of the KChIP2 gene is the primary determinant of the ventricular transmural gradient of I to in canine, ferret and human heart [30, 35] but not in rat heart. In rat, Kv4.2 level was assumed to be the main determinant of the gradient in I to expression [7, 35] and KChIP2 to be uniformly expressed across the left ventricular wall [14, 35]. Our study, however, shows quantitative differences in levels of KChIP2 mRNA across the rat ventricular wall. Interestingly, a recent study [42] described a transmural distribution of KChIP2 in the mouse heart, though previous studies had not. The small (30–50%) gradient in KChIP2 expression we were able to detect may be due to the sensitivity of our methodology, real-time RT-PCR versus RNAse mapping. Our observation that expression of mRNA for the transcription factor gene Irx5 is inversely related to Kv4.2 (that is, it is significantly higher in ENDO than EPI) is consistent with observations in mice, dog [5] and rats [34]. Similarly, the absence of a gradient of calcineurin A beta mRNA expression is in accord with findings in mice [36]. We did not investigate calcineurin A beta activity [36]. However, we have no evidence to suggest that the mRNA levels of any of these regulatory factors for I to were modified by voluntary exercise.
In addition, the ratio of EPI:ENDO Kv4.2 protein was similarly unaltered by exercise. Our Western blot analysis of Kv4.2 revealed two bands at ~79 and 74 kDa. These bands have been reported in other studies which included rat ventricular myocytes [4, 37, 43]. It has been suggested that the upper band represents Kv4.2 protein specifically trafficked to the cell membrane, whereas the lower band represents Kv4.2 protein that can exist at either intracellular or surface sites [37, 43]. We did not see an effect of training on either band nor on the combined levels of Kv4.2 protein.
There are several possible explanations for a change in I to current density in the absence of alterations in mRNA or Kv4.2 protein levels. mRNA measured by RT-PCR can include active (ribosome associated) and non-active populations which can influence translational efficiency [20] and alteration in post-translational trafficking can affect the number of channels at active sites on the sarcolemma [19]. Because we saw no change in the activation/inactivation of I to, suggesting no change in channel characteristics, and no differences in Kv4.2 protein expression, suggesting no change in channel number, we speculate that the reduction in TRN EPI I to is the result of a decrease in the number of functional channels in the membrane.
Implications of the findings of this study
There is evidence of T-wave abnormalities in some, otherwise normal, humans undertaking regular exercise. Unfortunately these features are common to conditions such as hypertrophic cardiomyopathy, which is associated with sudden cardiac death in young athletes [15, 21, 31, 32] thus complicating the cardiac screening of young athletes. Flattening or inversion of the T-wave of the ECG may be attributed to a reduction in the transmural gradient in APD, e.g. [38] which can be pro-arrhythmic, e.g. [1, 9, 45]. The clinical implications of such ECG changes in normal humans in response to exercise, are still under debate [15, 21, 31, 32] though evidence suggests they are benign. Thus, although some responses of regular exercise mimic the responses of pathological stimuli (e.g. cardiac hypertrophy and electrical remodelling) the signalling pathways and eventual outcomes of physiological and pathological stimuli are not usually the same. The cellular mechanisms behind these electrical changes are not well understood. It is therefore interesting that reduced EPI I to density and APD lengthening are seen in response to both voluntary exercise ([27] and this study) and other hypertrophic stimuli [2, 38, 44].
These data suggest that alteration of transient outward current plays an important role in exercise-induced electrical remodelling and that the rat model of voluntary exercise mimic some human responses and is therefore useful for the study of the cellular mechanisms underlying exercise-induced electrical remodelling.
References
Antzelevitch C, Fish J (2001) Electrical heterogeneity within the ventricular wall. Basic Res Cardiol 96:517–527
Bryant SM, Shipsey SJ, Hart G (1999) Normal regional distribution of membrane current density in rat left ventricle is altered in catecholamine-induced hypertrophy. Cardiovasc Res 42:391–401
Clark RB, Bouchard RA, Salinas-Stefanon E, Sanchez-Chapula J, Giles WR (1993) Heterogeneity of action potential waveforms and potassium currents in rat ventricle. Cardiovasc Res 27:1795–1799
Colinas O, Gallego M, Setien R, Lopez-Lopez JR, Perez-Garcia MT, Casis O (2006) Differential modulation of Kv4.2 and Kv4.3 channels by calmodulin-dependent protein kinase II in rat cardiac myocytes. Am J Physiol Heart Circ Physiol 291:H1978–H1987
Costantini DL, Arruda EP, Agarwal P, Kim KH, Zhu Y, Zhu W, Lebel M, Cheng CW, Park CY, Pierce SA, Guerchicoff A, Pollevick GD, Chan TY, Kabir MG, Cheng SH, Husain M, Antzelevitch C, Srivastava D, Gross GJ, Hui CC, Backx PH, Bruneau BG (2005) The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell 123:347–358
Davies LA, Hopkins PM, Boyett MR, Harrison SM (2000) Effects of halothane on the transient outward K(+) current in rat ventricular myocytes. Br J Pharmacol 131:223–230
Dixon JE, McKinnon D (1994) Quantitative analysis of potassium channel mRNA expression in atrial and ventricular muscle of rats. Circ Res 75:252–260
Eisele JC, Schaefer IM, Randel Nyengaard J, Post H, Liebetanz D, Bruel A, Muhlfeld C (2008) Effect of voluntary exercise on number and volume of cardiomyocytes and their mitochondria in the mouse left ventricle. Basic Res Cardiol 103:12–21
Fabritz L, Kirchhof P, Franz MR, Eckardt L, Monnig G, Milberg P, Breithardt G, Haverkamp W (2003) Prolonged action potential durations, increased dispersion of repolarization, and polymorphic ventricular tachycardia in a mouse model of proarrhythmia. Basic Res Cardiol 98:25–32
Fischer P, Hilfiker-Kleiner D (2007) Survival pathways in hypertrophy and heart failure: the gp130-STAT axis. Basic Res Cardiol 102:393–411
Fletcher GF, Balady G, Blair SN, Blumenthal J, Caspersen C, Chaitman B, Epstein S, Froelicher ES, Sivarajan Froelicher VF, Pina IL, Pollock ML (1996) Statement on exercise: benefits and recommendations for physical activity programs for all Americans: a statement for health professionals by the committee on exercise and cardiac rehabilitation of the council on clinical cardiology, American Heart Association. Circulation 94:857–862
Franz MR (1999) Current status of monophasic action potential recording: theories, measurements and interpretations. Cardiovasc Res 41:25–40
Gima K, Rudy Y (2002) Ionic current basis of electrocardiographic waveforms: a model study. Circ Res 90:889–896
Guo W, Li H, Aimond F, Johns DC, Rhodes KJ, Trimmer JS, Nerbonne JM (2002) Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res 90:586–593
Hart G (2003) Exercise-induced cardiac hypertrophy: a substrate for sudden death in athletes? Exp Physiol 88:639–644
Himmel HM, Wettwer E, Li Q, Ravens U (1999) Four different components contribute to outward current in rat ventricular myocytes. Am J Physiol 277:H107–H118
Jew KN, Olsson MC, Mokelke EA, Palmer BM, Moore RL (2001) Endurance training alters outward K+ current characteristics in rat cardiocytes. J Appl Physiol 90:1327–1333
Kemi OJ, Ellingsen O, Smith GL, Wisloff U (2008) Exercise-induced changes in calcium handling in left ventricular cardiomyocytes. Front Biosci 13:356–368
Kostova Z, Wolf DH (2003) For whom the bell tolls: protein quality control of the endoplasmic reticulum and the ubiquitin-proteasome connection. EMBO J 22:2309–2317
MacKay VL, Li X, Flory MR, Turcott E, Law GL, Serikawa KA, Xu XL, Lee H, Goodlett DR, Aebersold R, Zhao LP, Morris DR (2004) Gene expression analyzed by high-resolution state array analysis and quantitative proteomics: response of yeast to mating pheromone. Mol Cell Proteomics 3:478–489
Maron BJ, Pelliccia A (2006) The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation 114:1633–1644
McCrossan ZA, Billeter R, White E (2004) Transmural changes in size, contractile and electrical properties of SHR left ventricular myocytes during compensated hypertrophy. Cardiovasc Res 63:283–292
Meiry G, Reisner Y, Feld Y, Goldberg S, Rosen M, Ziv N, Binah O (2001) Evolution of action potential propagation and repolarization in cultured neonatal rat ventricular myocytes. J Cardiovasc Electrophysiol 12:1269–1277
Mokelke EA, Palmer BM, Cheung JY, Moore RL (1997) Endurance training does not affect intrinsic calcium current characteristics in rat myocardium. Am J Physiol 273:H1193–H1197
Moore RL, Korzick DH (1995) Cellular adaptations of the myocardium to chronic exercise. Prog Cardiovasc Dis 37:371–396
Moraska A, Deak T, Spencer RL, Roth D, Fleshner M (2000) Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 279:R1321–R1329
Natali AJ, Wilson LA, Peckham M, Turner DL, Harrison SM, White E (2002) Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes. J Physiol 541:863–875
Pandit SV, Clark RB, Giles WR, Demir SS (2001) A mathematical model of action potential heterogeneity in adult rat left ventricular myocytes. Biophys J 81:3029–3051
Patel SP, Campbell DL (2005) Transient outward potassium current, ‘Ito’, phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms. J Physiol 569:7–39
Patel SP, Campbell DL, Morales MJ, Strauss HC (2002) Heterogeneous expression of KChIP2 isoforms in the ferret heart. J Physiol 539:649–656
Pelliccia A, Maron BJ (2001) Athlete’s heart electrocardiogram mimicking hypertrophic cardiomyopathy. Curr Cardiol Rep 3:147–151
Pelliccia A, Maron BJ, Culasso F, Di Paolo FM, Spataro A, Biffi A, Caselli G, Piovano P (2000) Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation 102:278–284
Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE (1989) Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol 66:1250–1257
Rosati B, Grau F, McKinnon D (2006) Regional variation in mRNA transcript abundance within the ventricular wall. J Mol Cell Cardiol 40:295–302
Rosati B, Pan Z, Lypen S, Wang HS, Cohen I, Dixon JE, McKinnon D (2001) Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J Physiol 533:119–125
Rossow CF, Dilly KW, Santana LF (2006) Differential calcineurin/NFATc3 activity contributes to the Ito transmural gradient in the mouse heart. Circ Res 98:1306–1313
Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS (2003) A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem 278:36445–36454
Shipsey SJ, Bryant SM, Hart G (1997) Effects of hypertrophy on regional action potential characteristics in the rat left ventricle: a cellular basis for T-wave inversion? Circulation 96:2061–2068
Stones R, Calaghan SC, Billeter R, Harrison SM, White E (2007) Transmural variations in gene expression of stretch-modulated proteins in the rat left ventricle. Pflugers Arch 454:545–549
Stones R, Natali A, Billeter R, Harrison S, White E (2008) Voluntary exercise-induced changes in {beta}2-adrenoceptor signalling in rat ventricular myocytes. Exp Physiol 93:1065–1075
Taggart P, Sutton P, Opthof T, Coronel R, Kallis P (2003) Electrotonic cancellation of transmural electrical gradients in the left ventricle in man. Prog Biophys Mol Biol 82:243–254
Teutsch C, Kondo RP, Dederko DA, Chrast J, Chien KR, Giles WR (2007) Spatial distributions of Kv4 channels and KChip2 isoforms in the murine heart based on laser capture microdissection. Cardiovasc Res 73:739–749
Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR, Johnston D, Sweatt JD (2004) Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci 24:3643–3654
Volk T, Nguyen TH, Schultz JH, Faulhaber J, Ehmke H (2001) Regional alterations of repolarizing K+ currents among the left ventricular free wall of rats with ascending aortic stenosis. J Physiol 530:443–455
Voss F, Schoels W, Lue J, Bauer A, Katus HA, Becker R (2005) Regional differences in the dynamics of refractoriness in intact and hypertrophied in situ canine hearts. Basic Res Cardiol 100:433–438
Wittwer M, Fluck M, Hoppeler H, Muller S, Desplanches D, Billeter R (2002) Prolonged unloading of rat soleus muscle causes distinct adaptations of the gene profile. FASEB J 16:884–886
Yancey SL, Overton JM (1993) Cardiovascular responses to voluntary and treadmill exercise in rats. J Appl Physiol 75:1334–1340
Zhang H, Hancox JC (2004) In silico study of action potential and QT interval shortening due to loss of inactivation of the cardiac rapid delayed rectifier potassium current. Biochem Biophys ResCommun 322:693–699
Acknowledgments
This study was supported by The British Heart Foundation and The Wellcome Trust.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Stones, R., Billeter, R., Zhang, H. et al. The role of transient outward K+ current in electrical remodelling induced by voluntary exercise in female rat hearts. Basic Res Cardiol 104, 643–652 (2009). https://doi.org/10.1007/s00395-009-0030-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-009-0030-6