Abstract
Background
Dietary nitrate (NO3−) has been shown to be useful as an ergogenic aid with potential applications in health and disease (e.g., blood pressure control). However, there is no consensus about the effects of dietary NO3− or beetroot (BR) juice supplementation on cognitive function.
Objective
The aim of this study was to evaluate the effects of a single dose of a chewable BR-based supplement on cognitive performance.
Methods
A double-blind randomized placebo-controlled two-period crossover clinical trial was carried out based on the extension of the CONSORT guidelines for randomized crossover trials. A total of 44 participants (24 F; 20 M; 32.7 [12.5] years; 66.3 [9.0] kg; 170 [9.2] cm; 22.8 [1.4] kg/m2) were randomly allocated to receive first either four BR-based chewable tablets (BR-CT) containing 3 g of a Beta vulgaris extract (RedNite®) or four tablets of a placebo (maltodextrin). A 4-day washout period was used before crossover. Ninety minutes after ingestion of the treatments, a neuropsychological testing battery was administered in each period. The trial was registered at clinicaltrials.gov NCT05509075.
Results
Significant improvements with moderate effect size were found on memory consolidation at the short and long term only after BR-CT supplementation via the Rey Auditory Verbal Learning Test immediate (+ 20.69%) and delayed (+ 12.34%) recalls. Likewise, enhancement on both frontal lobe functions (+ 2.57%) and cognitive flexibility (+ 11.16%) were detected after BR-CT. There was no significant change (p < 0.05) on verbal memory of short-term digits, working memory and information processing speed. Mixed results were found on mood and anxiety through the Beck Depression Inventory-II (BDI-II) and the State-Trait Anxiety Inventory (STAI-Y1 and STAI-Y2); however, sequence and period effects were seen on STAI-Y2.
Conclusions
The acute administration of a chewable BR-based supplement improves certain aspects of cognitive function in healthy females and males, particularly memory capacity and frontal skills.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consumption of naturally nitrate- (NO3−) rich foods such as beetroot (BR), spinach, arugula or amaranth has shown a positive effect on health and disease [1]. The increased levels of blood NO3− after NO3− rich foods or supplements result in the augmentation of nitrite (NO2−) concentration and higher production of nitric oxide (NO) [2]. The synthesis of NO from NO3− is an alternative pathway to the canonical one that involves nitric oxide synthase (eNOS) using l-arginine as the main substrate [3]. NO3− from the diet have a first conversion into NO2− by the salivary microbiome which is then exposed to the low pH environment of the gastric acid and is reduced to NO. The significant increase in NO occurs through a process orchestrated by several tissues of the gastrointestinal tract, the blood, and the endothelium [4] (Fig. 1). It should be noted that the greatest conversion occurs in hypoxic conditions (as eNOS needs oxygen to be active) [3].
The nitrate/nitrite/nitric oxide (NO3−/NO2−/NO) pathway after dietary NO3− ingestion. Next to BR ingestion, oral microbiota on the posterior surface of the tongue is able to reduce NO3− to NO2− by means of their enzymatic machinery. The strict anaerobes Veillonella atypical and Veillonella dispar are the most important NO3− reducers; however, Actinomyces, Rothia, Prevotella, Neisseria, and Haermophilus are also present in the oral cavity. Even though this nonenzymatic reduction process continues in the stomach, where more NO2− and NO are produced due to the acid environment, a considerable amount of NO3− from blood (≈ 25%) is taken up by an electrogenic 2NO3−/H+ symporter called SLC17A5 (also known as sialin) in the salivary gland acinar cells [78]. Both dietary and saliva NO3−, and its reduced forms NO2− and NO, enter directly to systemic circulation after the absorption process in the stomach and intestine. Thus, the increase of NO3− and NO2− concentrations in blood allow the generation of NO by either enzymatic or non-enzymatic mechanisms (such as xanthine oxidoreductase, respiratory chain enzymes, aldehyde oxidase, methemoglobin formation, protons, etc.), especially under physiologic hypoxia and low pH [79]. Because of its short half-life (1–2 ms), once NO is produced in blood, it is broken down by hemoglobin or it can diffuse into the vascular smooth muscle cells or neurons and bind to guanylyl cyclase, which allows the allosteric activation of this last and subsequent cGMP production [80]. Here, cGMP acts as a second messenger and activates PKG, which in turn can modulate smooth muscle relaxation by several interlinked mechanisms: (i) activation of K+ channels leading to hyperpolarization; (ii) reduction of intracellular Ca2+ concentration; and (iii) activation of the myosin light-chain phosphatase [81]. Finally, NO3− is normally excreted in the urine by the kidneys. BP blood pressure, NO nitric oxide. Modified with permission from Bonilla et al. [4]
Importantly, dietary NO3− has shown to increase cerebral blood flow in humans [5]; therefore, NO could not only reach skeletal muscle, but also cross the blood–brain barrier and act on the central nervous system [6, 7]. Indeed, the main areas in which NO3− supplementation has been studied include those that obtain an ergogenic effect [8,9,10,11] or as regulators of blood pressure and cardiovascular health [4, 12, 13]. It is plausible that the non-enzymatic-dependent production of NO is responsible for most of the health and exercise performance benefits, although the presence of other secondary metabolites (e.g., betalains, oxalic acid, hydroxycinnamic acids) could also mediate the physiological response to NO3− rich foods [12]. It is necessary to point out that some effects of NO3− supplementation are a scientific controversy possibly due to the lack of standardization in the concentration (i.e., the amount of NO3− present in the extract), the dietary source, the timing of intake [14], the salivary oral microbiome [15, 16], and individual responses based on adaptations to stress conditions (e.g., physical exercise). Moreover, there are concerns related to potential toxicity, but expert consensus suggests that dietary NO3− supplementation up to ~ 16 mmol per day does not increase risk of cancer, methemoglobinemia hypotension, or renal injury [9]. Nowadays, it is clear that skeletal muscle concentration of NO3− and an optimal production of NO are both critical for healthy aging and disease management [17,18,19].
Several neurological diseases have been associated with the age-dependent decrease of cerebral blood flow [20, 21]. This has raised the potential of NO3− supplementation to slow the cognitive decline in elderly populations. Although BR supplementation has shown positive effects on perfusion to the brain [22, 23], the effects on cognitive performance continue being studied through the last years but remain unclear [24]. It has been reported that dietary NO3− might potentially improve cognitive performance in healthy adults [5] and in type-2 diabetes patients [25]. The systematic review performed by Stanaway et al. not only showed a lack of studies measuring cognitive performance-related variables (only 3 of 12 included studies), but also reported mixed findings [26]. Consistent with previous findings, a recent randomized, double-blind crossover trial showed only certain improvements of cognitive performance in the Stroop test, but not in choice reaction test or rapid visual information processing after BR supplementation [27]. The authors suggested that the positive effects of BR might be only present when a large degree of cognitive difficulty is imposed. Notwithstanding, it has been shown recently that acute dietary NO3− supplementation via red spinach extract or BR juice has no important effects on cognitive performance, in resistance-trained males or in healthy participants exercising at moderate or very high simulated altitudes, respectively. Currently, there is no consensus about the effects of dietary NO3− or BR supplementation on cognitive function in the elderly population [28,30,30] or in athletes [9]. Thus, the aim of this study was to assess the acute effects of a chewable BR-based supplement on cognitive performance (memory and executive function) using a neuropsychological battery of tests in apparently healthy female and male individuals.
Methods
Trial design
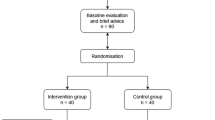
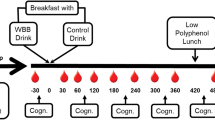
This study was a double-blind randomized placebo-controlled two-period crossover clinical trial to assess cognitive performance after the administration of a chewable BR-based supplement. The neuropsychological testing battery was applied at the end of each period. The experimental procedures were conducted following those established in the extension of the Consolidated Standards of Reporting Trials (CONSORT) for randomized crossover trials [31]. A 4-day washout period was used to reduce the carryover effect [32]. The study design is schematized in Fig. 2.
Participants
A total of 70 participants volunteered to participate in this clinical trial. Subjects were suitable for eligibility if they were: (i) apparently healthy; (ii) 18–60 years of age; (iii) non-smokers; (iv) without overt pathologies; (v) not taking drugs regularly; and (vi) committed to follow instructions of the research team at the testing time. The individuals were informed about the experimental protocol and all provided informed written consent before participating. The study protocol was approved by the Regional Ethical Committee (Center Italy Section) N12017052018.
Settings and location
The administration of the placebo or the active tablets and the application of the neuropsychological testing battery were performed within the Department of Medical and Surgical Sciences facilities at the University of Magna Grecia, Catanzaro Italy. The battery was administered to each participant in the same order and in two single test sessions. Parallel test forms were administered to verify the learning effect. All tests were provided by the same neuropsychologist (with more of than 10 years of experience).
Intervention
The participants that met the inclusion criteria were administered four chewable tablets with the same size, color, and shape of either active product or placebo 90 min before the neuropsychological test. The participants were asked to dissolve the tablets in the mouth (to favor the contact with the salivary microbiome) and to swallow saliva as many times as possible. The BR-based chewable tablets (BR-CT) contained 3 g of a Beta vulgaris extract (RedNite®, Enovate Biolife, Wilmington, DE) with standardized NO3− concentration range between 1.5 and 2.75% (equals to 45–82.5 mg of NO3−). NO3− supplementation with RedNite®-based products has already shown clinically significant effects on neuromuscular efficiency in healthy males [33]. The BR-CT also contains 200 mg of vitamin C and 100 mg betaine, both were used in those low doses to synergize the conversion of NO3− into NO avoiding its conversion in nitrosamine, as well as Citrus sinensis (blood orange) extract as flavoring, sucralose as sweetener, and excipients and stabilizers for the constitution of the tablet.
The placebo contained maltodextrin instead of the active components and had the same shape, color, and taste. The participants were requested to avoid food, not to rinse the mouth, not to engage in strenuous physical activity in the 2-h period before taking the chewable tablets, and to avoid stimulating substances (i.e., caffeine or theophylline) 6 h prior to the intervention. The formulations were produced by Laboratori Plants Group (Pace del Mela ME, Italy).
Outcomes
Neuropsychological assessment
The battery was administered to each participant in the same order and in two single test sessions and parallel forms of tests were administered to check the learning effect in a testing session lasting from 30 to 40 min. The psychologist was not informed about the treatment, the BR-CT and placebo were of the same color, and neither the subject nor the researcher knew which was the placebo and which was the BR-CT The following validated tests were administered 90 min after taking BR-CT or placebo:
-
The Rey Auditory Verbal Learning Test (RAVLT) which includes the assessment of immediate (RAVLT_I) and delayed recalls (RAVLT_D) to evaluate short-term and long-term verbal memory, respectively [34,35,36]. In this study, parallel forms of RAVLT's words were used (e.g., tenda, tamburo, etc., and violino, bastone, etc.).
-
The Digit Span Forward (Digit Span F) and Backward (Digit Span B) tasks to evaluate the memory span: the verbal memory of short-term digits and working memory standardized for Italian population [37, 38] (these are subsets of the Wechsler Adult Intelligence Scale-Fourth Edition [WAIS-IV] [39]).
-
The Symbol Digit Modality Test (SDMT) to measure attention and processing speed [40]. In this study, the parallel forms were used [41].
-
The Frontal Assessment Battery (FAB) for a fast screening of the main frontal skills such as abstract reasoning, mental flexibility, motor programming, executive control of action, and inhibitory control [42, 43].
-
The Controlled Oral Word Association Test (COWAT/FAS), used as a measure of lexical assets, tests the ability to access the lexicon and cognitive flexibility [44, 45]. In this study, parallel forms were used (i.e., letters F, A, S and E, G, L).
-
The self-assessment questionnaires to investigate mood included the 2nd edition of the Beck Depression Inventory (BDI-II) [46, 47] used to assess the depressive symptoms of the participants and the State–Trait Anxiety Inventory-Form Y1 and 2 (STAI-Y1; STAI-Y2) with 40 entries [48, 49]. The STAI provides scores for two scales (20 and 20) distinguishing between a person's status and trait anxiety levels. It should be noted that parallel forms were not used for mood questionnaires.
-
The Retrospective Prospective Memory Questionnaire (PRMQ) which is a self-report questionnaire that provides information for both the prospective (PRMQ-P) and retrospective (PRMQ-R) scales [50, 51]. These non-parallel tests were applied to investigate the perception of subjects in any modifications of cognitive functions [52].
Sample size
The sample size was calculated, based on the percentage change of the primary outcomes. Previous literature in adults has shown significant differences in cognitive performance tests between 5 and 12% when comparing BR supplementation versus placebo [22, 25]. Therefore, we used a minimum effect of interest equal to 9% (8 SD), a type I error rate (α) of 0.05 and with a power of 0.8, and obtained a sample size of ten per group. Considering our randomization ratio, we needed 40 participants for the BR-CT and 10 for placebo. To allow for attrition, 70 participants were enrolled.
Randomization and sequence generation
Unequal randomization (4:1 randomization of active to placebo) was used to give more power for pairwise comparisons and detect adverse events due to the larger sample size in the active group. In addition, unequal randomization allows more variables to be tested and better recruitment. A random allocation sequence was computer generated (https://www.randomizer.org/). We performed a two-period crossover with a treatment sequence AB:BA, which means that participants allocated to the AB study arm receive treatment A first, followed by treatment B, and vice versa in the BA arm. The AB/BA is not only usable, but also is considered the most efficient two-period two-treatment design [53]. To exclude a priori the carryover effects, we used a 4-day washout period that was set at three to four times the blood plasma elimination half-life for NO3− (5–8 h) [54, 55].
Blinding
This was a double-blinded clinical trial because participants and those assessing the outcomes were blinded to intervention. The placebo tablets were identical in size, shape, color and taste, but contained inactive ingredients (maltodextrin). Every person involved in the study received two cruets, marked with a code provided by the manufacturer and unknown to us and to the subjects, inside three tablets each. Both, the cruets and the tablets, were identical to make distinguishing between them impossible. Therefore, participants itself choose was ensured. The placebo formulation was manufactured by the Laboratori Plants Group (Pace del Mela ME, Italy).
Statistical methods
The descriptive statistics are expressed as mean and standard deviation (SD) unless otherwise indicated. Following the extension of CONSORT guidelines for randomized crossover trials [31], we based our analysis on paired data (within-participant comparison) using an intention-to-treat approach. As it has been recommended for crossover trials [56], and before estimating the treatment effects, the results were analyzed for each intervention in each period [57] and the sequence and period effects were estimated. It should be noted that the carryover effect was avoided by using a washout period of sufficient duration (4 days) [58].
Based on current recommendations to improve data analysis practices, we implemented an estimation approach following analytical procedures reported in previous articles published by the DBSS Research Divison [59, 60]. Estimation statistics helps to obtain more thoughtful interpretation and more balanced evaluation of evidence [61]. To determine statistical significance in the analysis of paired data, we examined the 95% confidence intervals (CIs) for the difference (Δ) between the placebo and BR-CT. If the 95% CI excludes zero, the difference will attain significance at the p < 0.05 level. Effect size was calculated as unbiased Cohen’s d (dunb), considering a result of ≤ 0.2 as a small, 0.5 as a moderate, ≥ 0.8 as a large effect, and ≥ 1.30 as a very large effect [62]. Estimation plots were generated to display the paired data at placebo and after BR-CT supplementation. Percentages of change were calculated according to the formula: (BR-CT − placebo)/placebo) × 100. To help with the planning and commissioning of future crossover trials, we also report the correlation coefficient for each primary outcome during the within-participant comparison. The same statistical procedure was performed in the paired-data analysis for each sequence. The sequence effect was estimated by comparing the means of the dependent variables in the AB and BA sequences.
In the analysis for each intervention in each period, we used the Yuen–Dixon test [63] with 20% trimmed means (μt) and 20% winsorized standard deviations (σw) as a robust statistical method for unequal-sized samples (e.g., BR-CT [n = 33] versus placebo [n = 11]). This robust statistics not only can be applied to factorial-type experimental designs, but also provide broader control of Type I error when variances are not equal [64]. A difference-in-differences (DID) analysis was performed to estimate the period effect by comparing changes in the outcome variables between each period. Statistical analyses were performed using the Exploratory Software for Confidence Intervals [65].
Results
Participant flow
After the call to participate, 70 participants were potentially eligible. However, 26 individuals were excluded from this study due to the presence of pathologies (n = 16), while others had concerns about taking the supplement and declined to participate (n = 10). Hence, 44 nonsmoking healthy participants (18–60 years of age) without overt pathologies completed this clinical trial. Figure 3 shows the CONSORT flow diagram modified for randomized crossover trial designs.
Baseline data
Table 1 resumes the characteristics of participants. The supplementation with BR-CT was well tolerated among all participants and there were no reported adverse effects, acute or 1 month after the study.
Outcomes and estimation
The results of the within-participant comparison (analysis on paired data) are expressed as Δ (SD) [95% CI]; dunb [95% CI] and presented in Table 2. The results on the RAVLT_D and RAVLT_I tests after BR-CT consumption showed higher statistically significant values with moderate effect size in comparison to placebo (12.34% and 20.69%, respectively). There were no significant differences between BR-CT and placebo, neither on Digit Span both forward (0.02 (1.19) [− 0.33, 0.38]; 0.021 [− 0.31, 0.35]) and backward (0.06 (1.10) [− 0.26, 0.40]; 0.069 [− 0.26, 0.40]), nor on the SDMT (− 4.43 (15.79) [− 9.23, 0.37]; 0.320 [− 0.67, 0.02]) tests. The FAB and COWAT/FAS tests were significantly improved with small effect size after BR-CT supplementation (2.57% and 11.16%, respectively). Although the results of the BDI test revealed significantly higher rates of depression after BR-CT compared to placebo (1.54 (3.63) [0.44, 2.64]; 0.261 [0.07, 0.45]), the STAI-Y1 and STAI-Y2 did not show significant differences on the anxiety levels between conditions. Finally, there were no differences in memory performance via the prospective, retrospective, and total PMRQ test (p > 0.05).
Figure 4 shows the estimation plots with raw data of each paired set of observations connected by a line to compare the placebo and BR-CT conditions. To complement the analysis, the changes for each sequence with the corresponding sequence effects are reported in Table 3. A higher number of variables showed significant differences in sequence AB. While BR-CT supplementation revealed significant improvements with large effect size on the RAVLT_D (– 6.12 (11.52) [– 10.2, – 2.0]; 0.740 [1.27, 0.23]), RAVLT_I (– 2.51 (4.07) [– 3.95, – 1.07]; 0.926 [1.51, 0.36]) and COWAT/FAS (– 6.27 (11.79) [– 10.4, – 2.09]; 0.733 [1.26, 0.23]) tests, the consumption of placebo showed better outcomes on SDMT (10.30 (18.51) [3.73, 16.86]; 0.775 [0.26, 1.31]) and less anxiety levels via BDI (– 2.36 (6.54) [– 4.68, – 0.04]; 0.473 [0.95, 0.008]). On the other hand, only two variables showed significant difference in sequence BA, both favoring the BR-CT condition: Digit Span B (0.90 (1.04) [0.20, 1.61]; 0.752 [0.14, 1.45]) and SDMT (13.18 (14.00) [3.77, 22.59]; 1.156 [0.25, 2.01]). There was a sequence effect by randomization in the AB/BA sequence only on STAI Y2 (p = 0.011). No other sequence effects were found.
Estimation plots examining the within-participant comparisons on the study variables. Paired data from placebo (maltodextrin) and experimental (BR-CT) conditions are shown as small circles joined by blue lines. The differences between the placebo and treatment means are plotted on a floating difference axis whose zero is aligned with the placebo mean. The filled pink triangle marks the difference on that axis and the 95% CI on that difference is displayed. The differences are shown as open triangles on the difference axis
The results of the robust analysis (ESt (MoEΔ) [95% CI]) for each intervention in each period with the corresponding period effects (DID [95% CI]; p value) are shown in Table 4. Significantly higher anxiety levels were found on the placebo condition through STAI Y2 (7.14 (6.74) [0.39, 13.8]); however, a period effect was found for this variable (– 12.3 [– 21.7, – 2.9]; 0.011). Figure 5 shows the estimation plots examining the effect of BR-CT on the study variables in each period. Finally, there was a direct effect of the treatment (BR-CT) only on long-term memory performance via RAVLT_D (p = 0.032).
Estimation plots examining the effect of BR-CT on the study variables. Individual participants from each group are shown. Removed data points are displayed as crosses, while retained points are red. The large circles with error bars represent each group trimmed mean with their 95% confidence intervals. The difference between the experimental (BR-CT) and placebo (maltodextrin) trimmed means is plotted on a floating difference axis. The filled red triangle marks the difference between groups on that axis and the 95% CI on that difference is displayed. BR-CT beetroot chewing tablets
Discussion
This double-blind randomized placebo-controlled crossover clinical trial aimed to evaluate the effects of a chewable NO3−-rich BR-based supplement on cognitive performance. We expected that the acute supplementation with a NO3−-rich product would have a positive impact on cognitive function in healthy individuals. We partially confirmed this initial hypothesis given that several, but not all neuropsychological tests showed significant difference between BR-CT and placebo conditions. Our results indicated a clinically significant improvement on both immediate (+ 20.69%) and delayed (+ 12.34%) memory capacity after BR-CT supplementation in comparison to the placebo condition. It is important to point out that the list of 15 words we administered with the RAVLT test was repeated five times, and at the testing time the participants had to repeat the words they remember. This process of reading the words five times put into action mechanisms of repetitive learning and memory consolidation at the short and long term that were enhanced by BR-CT supplementation. Notwithstanding, there were no significant changes (p > 0.05) on verbal and visuo-spatial short-term memory (digit span forward and backward). In addition, the lack of statistically significant variation on the PRMQ tests guarantees that there was no influence on the consciousness of carrying out a test. PRMQ is a self-report questionnaire to measure prospective and retrospective memory slips in everyday life. This test was properly included in the assessment to check the unconscious effects and the participants' perception with respect to any improvements in their performance. We should emphasize that the participants did not know when they would take the NO3−-rich BR-based supplement and when the placebo, and also that all were inexperienced in experimental research. Even though there was no significant improvement on information processing speed via SDMT, the results of the FAB test showed significant differences on the frontal lobe functions after BR-CT supplementation (+ 2.57%; dunb = 0.314). The executive, abstraction and re-elaboration skills are characteristics of the frontal area and are also fundamental in the research processes of the most suitable strategies for memorization processes [66, 67]. Moreover, our neuropsychological measure of verbal fluency (COWAT/FAS) presented a clinically significant improvement after the administration of the BR-CT supplement (+ 11.16%; dunb = 0.403). Finally, the other two tests concerning mood and anxiety revealed significant difference in BDI (p = 0.007), but not on STAI-Y1 and Y2 after BR-CT; nevertheless, the sequence and period effect that was found on STAI-Y2 makes clear that the condition might not have been a stable or certain between-subject variability for this test. Similar to other recent positive findings on cognitive reaction time and memory retrieval speed, our results showed that acute NO3− supplementation improves certain areas of cognition. The improvements in frontal skills as well as lexical and memory capacity are elements that confirm an enhancement in general cognitive capacity. Complementarily, the BR-CT supplement used in this study was well tolerated (it did not show any kind of undesirable or adverse effect) and even the palatability was appreciated. This latter aspect should not be underestimated, not only for what concerns the compliance in use, but also this has allowed a longer stay in the mouth with consequent greater exposure to the salivary microbiota, with the consequent possible greater conversion of NO3− into NO2− and absorption in the plasma circulation. It might be possible that the increase in cerebral blood flow [5], and thereby the higher nutrient supply, after the supplementation with the chewable NO3−-rich BR-based product might have impacted the cognitive performance. Several studies highlight the NO synthesis in the brain and its role in various neuronal functions, including learning and memory processes, cortical arousal, nociception, food intake, penile erection, yawning, blood vessel dilation, and the immune response [68, 69]. Multiple neuronal mechanisms might be involved in how NO appears to affect learning, short-term memory, and long-term memory which is associated with changes in behavior while mammals are learning and at various post-learning periods [70]. Numerous studies have shown that short-term memory and long-term memory represent separate processes [71, 72]. In principle, NO transmission may or may not affect learning behavior long-term memory or all these processe, and this could also be in line with the studies of neuronal plasticity, which affirm that it is no longer just a single circuit that is responsible for specific cognitive functions. Indeed, the discovery that NO and H2S participate as second messengers that influence visual working memory will lead to a paradigm shift in our understanding of working memory mechanisms and the organizational features of brain structures [73]. In general, our collective findings revealed improvements on memory ability and frontal lobe functions in humans, albeit the reliance on NO activation of the computational ability of the brain [70] deserves further investigation. It is important to underline that the psychodiagnostic tests used in this study, and which investigate cognitive functions, consist of a parallel form. In the first phase, a specific form was administered and in phase 2 the same test was administered, with the same psychometric structure but a different form, so that the learning effect could be controlled. To be certain that the learning was not due to a memory of the elements already learned before and, therefore, to the test but to an effect of the integrator. However, it is essential to remember that the psychodiagnostic tests used in this study, which investigate cognitive functions, are of the parallel "structure form". In the first phase, a specific structure form was administered, and in the second phase, the test was with the same psychometric structure but a different type of form, to control the learning effect. To be certain, the learning was not due to a memory of the elements already learned before and, therefore, to the test but to an effect of the supplement. For example, the words of the RAVLT that the participant heard in the first administration were not the same as those heard in the second administration. Finally, although we did not find significant influence on the results based on the level of education, the intake of coffee (more than two espresso coffees per day—about 50–70 mg of caffeine) or the sex of participants, future studies might emphasize these associations and study individual responses. It needs to be noted that our crossover design avoided problems of comparability of BR-CT and placebo groups regarding confounding variables (e.g., age and sex).
Limitations and strengths
The results of our study should be discussed in light of the following limitations and strengths. First, we did not measure the blood concentrations of NO3−, NO2−, or NO to ensure that there was a significant increase in this metabolite after BR-CT supplementation. Next, studies might evaluate if there is an actual and clinical difference between the chewable and drink versions of NO3− supplementation on cognitive function and exercise performance. Secondly, we did not use near-infrared spectroscopy to detect changes in cerebral blood flow as an indirect measure of brain activity. Third, we did not measure NO3− concentration in our BR-CT supplement to be sure the reported amount that has been relayed in literature for physiological benefits was given to the participants. However, RedNite®, (Enovate Biolife, Wilmington, DE) is a product with a range between 1.5 and 2.75% of standardized NO3− concentration (equal to 45–82.5 mg of NO3−). Last, the wide range of the participants age that could lead difference cognitive performance and the not counterbalanced administration of the supplement. However, the following strengths must be noted. The double-blinding and the crossover design allowed participants to be exposed to both treatments in similar conditions, eliminated between-subject variability, and gave more statistical power because of paired comparisons [74]. In fact, each participant served as his/her own control, which distinguishes from a conventional parallel-group trial [57]. Moreover, a neuropsychological battery with validated and well-recognized tests to evaluate cognitive function was applied by a neuropsychologist with more than 10 years of experience.
Future directions
Since NO3− from vegetables, whether cooked or uncooked, is absorbed very effectively in healthy human participants (absolute nitrate bioavailability ≈ 100%) [75], upcoming research should evaluate the effects of high NO3− diets on cognitive function for practical purposes. It is likely that there is an additive effect of dietary NO3− following repeated consumption of BR; consequently, future research should study the chronic effects of the chewable versions of NO3−-rich products with the corresponding assessment of BR-dependent microbiota changes in different ages throughout the life span [76]. Finally, a recent randomized crossover clinical trial by Jackson et al. [80] demonstrated that BR juice co-supplementation with apple and coffee berry phenolic acid-rich extracts increased oxygen saturation in the frontal cortex and reduced mental fatigue [77]. Thus, future studies might focus on evaluating the effects of dietary nitrate in combination with other nutrients or bioactive compounds (e.g., phenolic-rich extracts).
Conclusions
The acute administration of a chewable BR-based supplement improves certain aspects of cognitive function in healthy females and males, particularly, memory capacity and frontal skills. No significant changes were detected in both working memory and information processing speed after BR-CT supplementation. Although the chewable form of this BR-based supplement appeared to be safe and effective, more investigation in several population conditions is needed. The results of this study contribute to the body of evidence that focuses on the effects of NO3− supplementation on cognitive performance.
Data availability
The data that support the findings of this study are available on request from the corresponding author, RC.
References
Lundberg JO, Carlstrom M, Weitzberg E (2018) Metabolic effects of dietary nitrate in health and disease. Cell Metab 28(1):9–22. https://doi.org/10.1016/j.cmet.2018.06.007
Capper TE, Siervo M, Clifford T, Taylor G, Iqbal W, West D, Stevenson EJ (2022) Pharmacokinetic profile of incremental oral doses of dietary nitrate in young and older adults: a crossover randomized clinical trial. J Nutr 152(1):130–139. https://doi.org/10.1093/jn/nxab354
DeMartino AW, Kim-Shapiro DB, Patel RP, Gladwin MT (2019) Nitrite and nitrate chemical biology and signalling. Br J Pharmacol 176(2):228–245. https://doi.org/10.1111/bph.14484
Bonilla DA, Paipilla AF, Marin E, Vargas-Molina S, Petro JL, Perez-Idarraga A (2018) Dietary nitrate from beetroot juice for hypertension: a systematic review. Biomolecules. https://doi.org/10.3390/biom8040134
Wightman EL, Haskell-Ramsay CF, Thompson KG, Blackwell JR, Winyard PG, Forster J, Jones AM, Kennedy DO (2015) Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Physiol Behav 149:149–158. https://doi.org/10.1016/j.physbeh.2015.05.035
Garthwaite J (2019) NO as a multimodal transmitter in the brain: discovery and current status. Br J Pharmacol 176(2):197–211. https://doi.org/10.1111/bph.14532
Uray T, Empey PE, Drabek T, Stezoski JP, Janesko-Feldman K, Jackson T, Garman RH, Kim F, Kochanek PM, Dezfulian C (2019) Nitrite pharmacokinetics, safety and efficacy after experimental ventricular fibrillation cardiac arrest. Nitric Oxide 93:71–77. https://doi.org/10.1016/j.niox.2019.09.003
Senefeld JW, Wiggins CC, Regimbal RJ, Dominelli PB, Baker SE, Joyner MJ (2020) Ergogenic effect of nitrate supplementation: a systematic review and meta-analysis. Med Sci Sports Exerc 52(10):2250–2261. https://doi.org/10.1249/MSS.0000000000002363
Shannon OM, Allen JD, Bescos R, Burke L, Clifford T, Easton C, Gonzalez JT, Jones AM, Jonvik KL, Larsen FJ, Peeling P, Piknova B, Siervo M, Vanhatalo A, McGawley K, Porcelli S (2022) Dietary inorganic nitrate as an ergogenic aid: an expert consensus derived via the modified Delphi technique. Sports Med. https://doi.org/10.1007/s40279-022-01701-3
Wong TH, Sim A, Burns SF (2021) The effect of beetroot ingestion on high-intensity interval training: a systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu13113674
Silva KVC, Costa BD, Gomes AC, Saunders B, Mota JF (2022) Factors that moderate the effect of nitrate ingestion on exercise performance in adults: a systematic review with meta-analyses and meta-regressions. Adv Nutr. https://doi.org/10.1093/advances/nmac054
Bahadoran Z, Mirmiran P, Kabir A, Azizi F, Ghasemi A (2017) The nitrate-independent blood pressure-lowering effect of beetroot juice: a systematic review and meta-analysis. Adv Nutr 8(6):830–838. https://doi.org/10.3945/an.117.016717
Alsulayyim AS, Alasmari AM, Alghamdi SM, Polkey MI, Hopkinson NS (2021) Impact of dietary nitrate supplementation on exercise capacity and cardiovascular parameters in chronic respiratory disease: a systematic review and meta-analysis. BMJ Open Respir Res. https://doi.org/10.1136/bmjresp-2021-000948
Shannon OM, Easton C, Shepherd AI, Siervo M, Bailey SJ, Clifford T (2021) Dietary nitrate and population health: a narrative review of the translational potential of existing laboratory studies. BMC Sports Sci Med Rehabil 13(1):65. https://doi.org/10.1186/s13102-021-00292-2
Tribble GD, Angelov N, Weltman R, Wang BY, Eswaran SV, Gay IC, Parthasarathy K, Dao DV, Richardson KN, Ismail NM, Sharina IG, Hyde ER, Ajami NJ, Petrosino JF, Bryan NS (2019) Frequency of tongue cleaning impacts the human tongue microbiome composition and enterosalivary circulation of nitrate. Front Cell Infect Microbiol 9:39. https://doi.org/10.3389/fcimb.2019.00039
Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, Morris A (2017) Enterosalivary nitrate metabolism and the microbiome: intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med 105:48–67. https://doi.org/10.1016/j.freeradbiomed.2016.12.015
Karwowska M, Kononiuk A (2020) Nitrates/nitrites in food-risk for nitrosative stress and benefits. Antioxidants (Basel). https://doi.org/10.3390/antiox9030241
Chen L, Zhu Y, Hu Z, Wu S, Jin C (2021) Beetroot as a functional food with huge health benefits: antioxidant, antitumor, physical function, and chronic metabolomics activity. Food Sci Nutr 9(11):6406–6420. https://doi.org/10.1002/fsn3.2577
Piknova B, Schechter AN, Park JW, Vanhatalo A, Jones AM (2022) Skeletal muscle nitrate as a regulator of systemic nitric oxide homeostasis. Exerc Sport Sci Rev 50(1):2–13. https://doi.org/10.1249/JES.0000000000000272
Zhang H, Wang Y, Lyu D, Li Y, Li W, Wang Q, Li Y, Qin Q, Wang X, Gong M, Jiao H, Liu W, Jia J (2021) Cerebral blood flow in mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Ageing Res Rev 71:101450. https://doi.org/10.1016/j.arr.2021.101450
Mokhber N, Shariatzadeh A, Avan A, Saber H, Babaei GS, Chaimowitz G, Azarpazhooh MR (2021) Cerebral blood flow changes during aging process and in cognitive disorders: a review. Neuroradiol J 34(4):300–307. https://doi.org/10.1177/19714009211002778
Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, Gilchrist M, Winyard PG, Jones AM (2013) Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol 304(2):R73-83. https://doi.org/10.1152/ajpregu.00406.2012
Presley TD, Morgan AR, Bechtold E, Clodfelter W, Dove RW, Jennings JM, Kraft RA, King SB, Laurienti PJ, Rejeski WJ, Burdette JH, Kim-Shapiro DB, Miller GD (2011) Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide 24(1):34–42. https://doi.org/10.1016/j.niox.2010.10.002
Horiuchi M, Rossetti GMK, Oliver SJ (2021) The role of dietary nitrate supplementation in neurovascular function. Neural Regen Res 16(7):1419–1420. https://doi.org/10.4103/1673-5374.300993
Aliahmadi M, Amiri F, Bahrami LS, Hosseini AF, Abiri B, Vafa M (2021) Effects of raw red beetroot consumption on metabolic markers and cognitive function in type 2 diabetes patients. J Diabetes Metab Disord 20(1):673–682. https://doi.org/10.1007/s40200-021-00798-z
Stanaway L, Rutherfurd-Markwick K, Page R, Ali A (2017) Performance and health benefits of dietary nitrate supplementation in older adults: a systematic review. Nutrients. https://doi.org/10.3390/nu9111171
Stanaway L, Rutherfurd-Markwick K, Page R, Wong M, Jirangrat W, Teh KH, Ali A (2019) Acute supplementation with nitrate-rich beetroot juice causes a greater increase in plasma nitrite and reduction in blood pressure of older compared to younger adults. Nutrients. https://doi.org/10.3390/nu11071683
Haynes JT, Townsend JR, Aziz MA, Jones MD, Littlefield LA, Ruiz MD, Johnson KD, Gonzalez AM (2021) Impact of red spinach extract supplementation on bench press performance, muscle oxygenation, and cognitive function in resistance-trained males. Sports. https://doi.org/10.3390/sports9060077
Shannon OM, Duckworth L, Barlow MJ, Deighton K, Matu J, Williams EL, Woods D, Xie L, Stephan BCM, Siervo M, O’Hara JP (2017) Effects of dietary nitrate supplementation on physiological responses, cognitive function, and exercise performance at moderate and very-high simulated altitude. Front Physiol 8:401. https://doi.org/10.3389/fphys.2017.00401
Ozawa H, Miyazawa T, Miyazawa T (2021) Effects of dietary food components on cognitive functions in older adults. Nutrients. https://doi.org/10.3390/nu13082804
Dwan K, Li T, Altman DG, Elbourne D (2019) CONSORT 2010 statement: extension to randomised crossover trials. BMJ 366:l4378. https://doi.org/10.1136/bmj.l4378
Li T, Yu T, Hawkins BS, Dickersin K (2015) Design, analysis, and reporting of crossover trials for inclusion in a meta-analysis. PLoS ONE 10(8):e0133023. https://doi.org/10.1371/journal.pone.0133023
Flanagan SD, Looney DP, Miller MJ, DuPont WH, Pryor L, Creighton BC, Sterczala AJ, Szivak TK, Hooper DR, Maresh CM, Volek JS, Ellis LA, Kraemer WJ (2016) The effects of nitrate-rich supplementation on neuromuscular efficiency during heavy resistance exercise. J Am Coll Nutr 35(2):100–107. https://doi.org/10.1080/07315724.2015.1081572
Carlesimo GA, De Risi M, Monaco M, Costa A, Fadda L, Picardi A, Di Gennaro G, Caltagirone C, Grammaldo L (2014) Normative data for measuring performance change on parallel forms of a 15-word list recall test. Neurol Sci 35(5):663–668. https://doi.org/10.1007/s10072-013-1573-8
Carlesimo GA, Caltagirone C, Gainotti G (1996) The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 36(6):378–384. https://doi.org/10.1159/000117297
Smirni D, Smirni P, Di Martino G, Cipolotti L, Oliveri M, Turriziani P (2018) Standardization and validation of a parallel form of the verbal and non-verbal recognition memory test in an Italian population sample. Neurol Sci 39(8):1391–1399. https://doi.org/10.1007/s10072-018-3433-z
Monaco M, Costa A, Caltagirone C, Carlesimo GA (2013) Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol Sci 34(5):749–754. https://doi.org/10.1007/s10072-012-1130-x
Varela JL, Ord AS, Phillips JI, Shura RD, Sautter SW (2022) Preliminary evidence for digit span performance validity indicators within the neuropsychological assessment battery. Appl Neuropsychol Adult. https://doi.org/10.1080/23279095.2022.2076602
Drozdick LW, Raiford SE, Wahlstrom D, Weiss LG (2018) The Wechsler Adult Intelligence Scale—Fourth Edition and the Wechsler Memory Scale—Fourth Edition. In: Flanagan DP, McDonough EM (eds) Contemporary intellectual assessment: theories, tests, and issues. The Guilford Press, New York, pp 486–511
Carone DA (2007) E. Strauss, E. M. S. Sherman, & O. Spreen, A compendium of neuropsychological tests: administration, norms, and commentary. Appl Neuropsychol 14(1):62–63. https://doi.org/10.1080/09084280701280502
Amodio P, Wenin H, Del Piccolo F, Mapelli D, Montagnese S, Pellegrini A, Musto C, Gatta A, Umilta C (2002) Variability of trail making test, symbol digit test and line trait test in normal people. A normative study taking into account age-dependent decline and sociobiological variables. Aging Clin Exp Res 14(2):117–131. https://doi.org/10.1007/BF03324425
Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML, Forapani E, Russo A, Nichelli P (2005) The Frontal Assessment Battery (FAB): normative values in an Italian population sample. Neurol Sci 26(2):108–116. https://doi.org/10.1007/s10072-005-0443-4
Aiello EN, Esposito A, Gramegna C, Gazzaniga V, Zago S, Difonzo T, Appollonio IM, Bolognini N (2022) The Frontal Assessment Battery (FAB) and its sub-scales: validation and updated normative data in an Italian population sample. Neurol Sci 43(2):979–984. https://doi.org/10.1007/s10072-021-05392-y
Costa A, Bagoj E, Monaco M, Zabberoni S, De Rosa S, Papantonio AM, Mundi C, Caltagirone C, Carlesimo GA (2014) Standardization and normative data obtained in the Italian population for a new verbal fluency instrument, the phonemic/semantic alternate fluency test. Neurol Sci 35(3):365–372. https://doi.org/10.1007/s10072-013-1520-8
Patterson J (2018) Controlled Oral Word Association Test. In: Kreutzer JS, DeLuca J, Caplan B (eds) Encyclopedia of clinical neuropsychology. Springer, Cham, pp 958–961.https://doi.org/10.1007/978-3-319-57111-9_876
Altieri M, Trojano L, Gallo A, Santangelo G (2020) The relationships between cognitive reserve and psychological symptoms: a cross-sectional study in healthy individuals. Am J Geriatr Psychiatry 28(4):404–409. https://doi.org/10.1016/j.jagp.2019.07.017
Beck AT, Steer RA, Ball R, Ranieri W (1996) Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 67(3):588–597. https://doi.org/10.1207/s15327752jpa6703_13
Spielberger CD (2010) State-Trait Anxiety Inventory. In: The Corsini encyclopedia of psychology. https://doi.org/10.1002/9780470479216.corpsy0943
Ilardi CR, Gamboz N, Iavarone A, Chieffi S, Brandimonte MA (2021) Psychometric properties of the STAI-Y scales and normative data in an Italian elderly population. Aging Clin Exp Res 33(10):2759–2766. https://doi.org/10.1007/s40520-021-01815-0
Smith G, Della Sala S, Logie RH, Maylor EA (2000) Prospective and retrospective memory in normal ageing and dementia: a questionnaire study. Memory 8(5):311–321. https://doi.org/10.1080/09658210050117735
Crawford JR, Smith G, Maylor EA, Della Sala S, Logie RH (2003) The Prospective and Retrospective Memory Questionnaire (PRMQ): normative data and latent structure in a large non-clinical sample. Memory 11(3):261–275. https://doi.org/10.1080/09658210244000027
Georgiev G (2020) Sample size calculator. https://www.gigacalculator.com/calculators/power-sample-size-calculator.php. Accessed 16 Apr 2021
Matthews JN, Henderson R (2013) Two-period, two-treatment crossover designs subject to non-ignorable missing data. Biostatistics 14(4):626–638. https://doi.org/10.1093/biostatistics/kxt009
Wagner DA, Schultz DS, Deen WM, Young VR, Tannenbaum SR (1983) Metabolic fate of an oral dose of 15N-labeled nitrate in humans: effect of diet supplementation with ascorbic acid. Cancer Res 43(4):1921–1925
Kadach S, Piknova B, Black MI, Park JW, Wylie LJ, Stoyanov Z, Thomas SM, McMahon NF, Vanhatalo A, Schechter AN, Jones AM (2022) Time course of human skeletal muscle nitrate and nitrite concentration changes following dietary nitrate ingestion. Nitric Oxide 121:1–10. https://doi.org/10.1016/j.niox.2022.01.003
Lim CY, In J (2021) Considerations for crossover design in clinical study. Korean J Anesthesiol 74(4):293–299. https://doi.org/10.4097/kja.21165
Wellek S, Blettner M (2012) On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int 109(15):276–281. https://doi.org/10.3238/arztebl.2012.0276
Martin OA, Teste FP (2022) A call for changing data analysis practices: from philosophy and comprehensive reporting to modeling approaches and back. Plant Soil. https://doi.org/10.1007/s11104-022-05329-0
Bonilla DA, Kreider RB, Petro JL, Romance R, Garcia-Sillero M, Benitez-Porres J, Vargas-Molina S (2021) Creatine enhances the effects of cluster-set resistance training on lower-limb body composition and strength in resistance-trained men: a pilot study. Nutrients. https://doi.org/10.3390/nu13072303
Cannataro R, Cione E, Gallelli L, Marzullo N, Bonilla DA (2020) Acute effects of supervised making weight on health markers, hormones and body composition in muay Thai fighters. Sports. https://doi.org/10.3390/sports8100137
Calin-Jageman RJ, Cumming G (2019) Estimation for better inference in neuroscience. eNeuro. https://doi.org/10.1523/ENEURO.0205-19.2019
Rosenthal JA (1996) Qualitative descriptors of strength of association and effect size. J Soc Serv Res 21(4):37–59. https://doi.org/10.1300/J079v21n04_02
Algina J, Keselman HJ, Penfield RD (2005) An alternative to Cohen’s standardized mean difference effect size: a robust parameter and confidence interval in the two independent groups case. Psychol Methods 10(3):317–328. https://doi.org/10.1037/1082-989X.10.3.317
Caldwell AR, Cheuvront SN (2019) Basic statistical considerations for physiology: the journal temperature toolbox. Temperature (Austin) 6(3):181–210. https://doi.org/10.1080/23328940.2019.1624131
Cumming G (2013) Understanding the new statistics; effect sizes, confidence intervals, and meta-analysis. 1st edn. Routledge, New York. https://doi.org/10.4324/9780203807002
Muller NCJ, Kohn N, van Buuren M, Klijn N, Emmen H, Berkers R, Dresler M, Janzen G, Fernandez G (2021) Differences in executive abilities rather than associative processes contribute to memory development. Hum Brain Mapp 42(18):6000–6013. https://doi.org/10.1002/hbm.25665
Capper T (2019) Ageing, dietary nitrate and whole beetroot consumption: acute and long-term effects on metabolic, vascular and cognitive function. Newcastle University, Newcastle
Garthwaite J, Boulton CL (1995) Nitric oxide signaling in the central nervous system. Annu Rev Physiol 57:683–706. https://doi.org/10.1146/annurev.ph.57.030195.003343
Susswein AJ, Katzoff A, Miller N, Hurwitz I (2004) Nitric oxide and memory. Neuroscientist 10(2):153–162. https://doi.org/10.1177/1073858403261226
Paul V, Ekambaram P (2011) Involvement of nitric oxide in learning & memory processes. Indian J Med Res 133:471–478
Milner B, Squire LR, Kandel ER (1998) Cognitive neuroscience and the study of memory. Neuron 20(3):445–468. https://doi.org/10.1016/s0896-6273(00)80987-3
Botzer D, Markovich S, Susswein AJ (1998) Multiple memory processes following training that a food is inedible in Aplysia. Learn Mem 5(3):204–219
Zars T (2017) Working memory: it’s a gas, gas. Gas Curr Biol 27(5):R179–R181. https://doi.org/10.1016/j.cub.2017.01.005
Reed JF 3rd (2004) Analysis of two-treatment, two-period crossover trials in emergency medicine. Ann Emerg Med 43(1):54–58. https://doi.org/10.1016/s0196-0644(03)00661-9
van Velzen AG, Sips AJ, Schothorst RC, Lambers AC, Meulenbelt J (2008) The oral bioavailability of nitrate from nitrate-rich vegetables in humans. Toxicol Lett 181(3):177–181. https://doi.org/10.1016/j.toxlet.2008.07.019
Capper TE, Houghton D, Stewart CJ, Blain AP, McMahon N, Siervo M, West DJ, Stevenson EJ (2020) Whole beetroot consumption reduces systolic blood pressure and modulates diversity and composition of the gut microbiota in older participants. NFS J 21:28–37. https://doi.org/10.1016/j.nfs.2020.08.001
Jackson PA, Wightman EL, Veasey R, Forster J, Khan J, Saunders C, Mitchell S, Haskell-Ramsay CF, Kennedy DO (2020) A randomized, crossover study of the acute cognitive and cerebral blood flow effects of phenolic, nitrate and botanical beverages in young, healthy humans. Nutrients. https://doi.org/10.3390/nu12082254
Qu XM, Wu ZF, Pang BX, Jin LY, Qin LZ, Wang SL (2016) From nitrate to nitric oxide: the role of salivary glands and oral bacteria. J Dent Res 95(13):1452–1456. https://doi.org/10.1177/0022034516673019
Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC (2013) Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol 591(2):547–557. https://doi.org/10.1113/jphysiol.2012.243121
Yuste JE, Tarragon E, Campuzano CM, Ros-Bernal F (2015) Implications of glial nitric oxide in neurodegenerative diseases. Front Cell Neurosci 9:322. https://doi.org/10.3389/fncel.2015.00322
Velmurugan S, Kapil V, Ghosh SM, Davies S, McKnight A, Aboud Z, Khambata RS, Webb AJ, Poole A, Ahluwalia A (2013) Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex. Free Radic Biol Med 65:1521–1532. https://doi.org/10.1016/j.freeradbiomed.2013.06.031
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows—RC, MGV, BI, DAB and EC: designed the study; LG and MGV: recruited participants and acquired the data with help from GDS; DAB: formal analysis of the data; RC, DAB, EC, and MGV: wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
D.A.B. serves as science product manager for MTX Corporation®, a company that produces, distributes, sells, and does research on dietary supplements (including NO3−-rich products) in Europe, has acted as a scientific consultant for MET-Rx and Healthy Sports in Colombia, and has received honoraria for speaking about nutrient supplements at international conferences. He is also a member of the “Creatine for Health” initiative for Alzchem Group AG. R.C. is employed by Galascreen Srl., Vita Vegan Excellence, and BlowC. The other authors declare no conflicts of interest. Laboratori Plants Group and De Lorenzo Srl had no other role in the trial whatsoever except for supplying and commercialize the chewable tablets, respectively.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vaccaro, M.G., Innocenti, B., Cione, E. et al. Acute effects of a chewable beetroot-based supplement on cognitive performance: a double-blind randomized placebo-controlled crossover clinical trial. Eur J Nutr 63, 303–321 (2024). https://doi.org/10.1007/s00394-023-03265-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03265-y