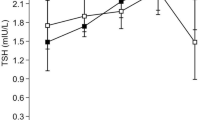
Abstract
Purpose
Populations following a plant-based diet may be at particular risk of thyroid dysfunction due to low iodine and selenium intakes. The main purpose was to assess thyroid function and urinary concentration of iodine, selenium, and arsenic, in subjects following a vegan, lacto-ovo vegetarian, or pescatarian diet.
Methods
In Norway, a country without mandatory dietary iodine fortification, 205 adults, following vegan (n = 115), lacto-ovo vegetarian (n = 55) and pescatarian diet (n = 35) were included. Thyroglobulin (Tg), thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), free thyroxine (fT4), and serum anti-TPO (S-anti-TPO) were measured in a venous blood sample and concentrations of iodine (UIC), creatinine (UCC), selenium, and arsenic were measured from single spot urine samples.
Results
Subclinical hypothyroidism (TSH > 4.0 mU/L) was observed in 3% of subjects. The overall median (p25, p75) Tg was 17 (9, 30) µg/L and vegans had higher Tg compared to pescatarians. Vegans not consuming iodine-containing supplements (n = 43) had higher Tg, than supplement users (n = 72), 27 (11, 44) vs. 16 (8, 25) µg/L and higher fT4, 16 (15, 17) vs. 15 (14, 17) pmol/L, respectively. The overall median UIC was 57 (28, 130) µg/L, all dietary groups had median UIC below WHO thresholds. Median urinary selenium and arsenic concentration was 13 (6, 22) and 3 (2, 8) µg/L, respectively.
Conclusion
The prevalence of subclinical hypothyroidism was low and fT4 and fT3 were within the normal range for all dietary groups. Vegans had significantly increased Tg compared to pescatarians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iodine is an essential micronutrient needed for the synthesis of thyroid hormones triiodothyronine (T3) and thyroxine (T4) and adequate production is required for growth, development, and regulation of the metabolism [1]. Mild to moderate iodine deficiency is widespread among adults in Europe [2], including Norway [3, 4]. Globally, the iodization of table salt is the main strategy to prevent iodine deficiency disorders and iodized salt is the primary dietary source of iodine in countries with mandatory iodine fortification policy [5]. Norway has not implemented salt iodization and low iodine intake has been reported among women of fertile age, pregnant and breastfeeding women, infants who are exclusively breastfed, elderly, vegans, and immigrants [4]. In Norway, animal source foods such as cow’s milk and yoghurt, eggs (due to iodine fortification of fodder), and marine fish are the main dietary iodine sources. WHO recommend assessment of median urinary iodine concentration (UIC) as an indicator for iodine status in a population [5]. In addition to UIC, thyroglobulin (Tg) can also serve as a marker of iodine deficiency and altered thyroid activity. When iodine intakes decrease, iodine stores in the thyroid gland diminish [6]. Tg, a protein synthesized by the thyroid cells, increases thyroid activity to maintain thyroid hormone concentrations within the normal range [7, 8].
People adhering to a plant-based diet can be categorized as vegans (no intake of animal source foods) lacto-ovo vegetarian (intake of milk/dairy products, cheese and/or eggs) or pescatarian (intake of milk/dairy products, cheese and/or eggs, in addition to fish/shellfish/fish products). Plant-based diets are typically associated with low iodine intakes compared to omnivore diets [9], unless iodine-containing supplements or microalgae is consumed. The iodine content in macroalgae may, however, vary widely both between and within species, and some products contain excessive amounts of iodine [10], and may be an unreliable iodine source [11]. Plant-based milk and dairy products have low iodine content, unless they are fortified with iodine [12, 13].
The synthesis of thyroid hormones is catalyzed by thyroid peroxidase (TPO) which uses selenium as a cofactor. Selenium is an essential element that is required for selenocysteine synthesis and production of selenoproteins [14]. Animal source foods such as dairy products, meat and seafoods have high selenium concentration, and for plant-foods, the concentration of selenium depend on the selenium level in soil. Lower selenium status has been reported in vegetarians and vegans as compared to omnivores in epidemiological studies [15]. Urine is the main route of excretion of selenium, mainly in the form of selenosugar [16] and have been considered as a useful marker of recent selenium intake [17] and a biomarker of selenium status of a population [18].
Inorganic arsenic has been found to inhibit TPO in vitro and arsenic exposure has been associated with thyroid dysfunction. Arsenic is an element that can exist in various chemical forms, with inorganic arsenic classified as carcinogenic whereas the organic form arsenobetaine is regarded non-toxic, in addition to many chemical forms not yet fully characterized for their toxicity [19]. In a Norwegian randomized controlled trial, ingestion of seafood rich in total arsenic was associated with an increase in thyroid-stimulating hormone (TSH) [20]. Most arsenic compounds present in food items are excreted in urine with a half time generally of a few days, and therefore, measurement of total urinary arsenic has been used as a biomarker of arsenic exposure [21, 22].
Since the most important dietary iodine sources in Norway are found in animal source foods, individuals following a plant-based diet are at particular risk of iodine deficiency [23], but the impact on thyroid function is uncertain. Thus, the objective of this study is to assess thyroid function and urinary iodine, creatinine, selenium, and arsenic concentration in subjects adhering to a vegan, lacto-ovo vegetarian, and pescatarian diet.
Materials and methods
Subjects and study design
Recruitment of participants in this cross-sectional study was conducted through convenience sampling and snowball sampling from September to November 2019, in the Oslo area in Norway. Participants were recruited through social media in closed Facebook groups and in online vegan and vegetarian forums. Details about the recruitment method and inclusion criteria have previously been described [23]. Study inclusion criteria were: (1) consumption of a vegan, lacto-ovo vegetarian or pescatarian diet for a minimum of 6 months; (2) age 18 years or older; (3) not currently pregnant or lactating; (4) no current use of thyroid medication.
We recruited 236 participants, and 29 people did not meet the inclusion criteria. In addition, two participants were excluded from the data analysis because of thyroid medication use and occasional meat consumption. Thus, the final sample consisted of 205 subjects, of which 115 vegans, 55 lacto-ovo vegetarians and 35 pescatarians. Participants who had provided study consent filled out an electronic questionnaire which consisted of background characteristics (age, anthropometric measures (height and weight), marital status, occupational status, educational level, smoking habits, country of birth, language, duration of adherence to vegan/lacto-ovo vegetarian/pescatarian diet) and foods included in the diet the previous 6 months (used to categorize participants into the different diet groups). The participants also conducted a 24-h dietary recall and a food frequency questionnaire (FFQ) for assessment of dietary intakes the previous 4 weeks (dietary iodine intakes are not presented in this paper, as these data have previously been published [23].
Assessment of dietary supplement use and macroalgae use
Iodine supplement use was assessed both by 24 h and habitual intake the previous 4 weeks (FFQ). The participants reported the name of the supplement, brand and amount used during the previous 24 h. By habitual intake, supplement consumption was reported as frequency per week, e.g., if a supplement contained 150 µg, and if taken four times a week, the contribution was estimated to be (50 µg × 4/7) 86 µg/day. Selenium-containing dietary supplements were also obtained from a FFQ in the electronic questionnaire in the same way as for iodine. For assessment of consumption of macroalgae, a dichotomous variable (no/yes) was used, if yes, participants reported type and amount (gram) used the previous 24 h and the habitual use the past 4 weeks. The types of macroalgae reported were Sugar kelp (Saccharina latissimi), Bladder wrack (Fucus vesiculosus), Wakame (Undaria pinnatifida), Kombu (Laminaria japonica and Saccharina japonica), Dulse (Palmaria palmata) and Laver (Porphyra spp.), with more details described in previous work [23].
Determination of concentrations of elements in urine
All participants (n = 205) provided one non-fasting spot urine sample collected non-fasted at random times throughout the day, in a labeled 100 mL Vacuette® urine beaker (Greiner Bio-One, Kremsmünster, Austria). A subsample of urine was transferred into a 9.5 mL Vacuette® urine tube (Greiner Bio-One, Kremsmünster, Austria) and immediately put to storage at 2–4 °C before freezing at – 80 °C until analyses. The urine concentrations of iodine, selenium, and arsenic were measured at the Norwegian University of Life Science, Faculty of Environmental Science and Natural Resource Management. The analysis was performed using an alkaline sample preparation and subsequent quantitative determination using Inductively Coupled Plasma Mass Spectrometry ICP-MS [23]. An aliquot of 1 mL of urine was transferred into 15 mL pp centrifuge tubes (Sarstedt, Nümbrecht, Germany) by means of 100–5000 µL electronic pipette (Biohit, Helsinki, Finland) and diluted to 10 mL adding an alkaline solution (BENT), containing 4% (weight (w)/volume(V) 1-Butanol and 0.1% (w/V) H4EDTA, 2% (w/V) NH4OH, and 0.1% (w/V) TritonTM X-100. Method blank samples and samples of standard reference material (SRM) were prepared following the same procedures. Reagent of analytical grade or better and deionized water (18 MΩ) were used throughout. The samples were analyzed for iodine, selenium, and arsenic concentration using an Agilent 8800 ICP-QQQ (Triple Quadruple Inductively Coupled Plasma Mass Spectrometer, Agilent Technologies, Hachioji, Japan) using oxygen as a reaction gas. The concentration of iodine was determined by measuring 127I isotope. Simultaneously, the concentration of selenium in urine was determined on by measuring the mass shift from 78 to 94, while arsenic in urine was determined using a mass shift from 75 to 91. The limits of detection (LOD) and limits of quantification (LOQ) were calculated by multiplying the standard deviation of the method blank samples (n = 10) by three and ten, respectively. The obtained LOD and LOQ were 0.3 µg/L and 0.92 µg/L for iodine, 0.06 µg/L and 0.2 µg/L for selenium and 0.01 µg/L and 0.05 µg/L for arsenic, respectively. To check for method accuracy, two reference materials of urine (Sero AS, Billingstad, Norway) were analyzed; each with value assignment established in accordance with the Essential Requirements of the IVD Directive1 98/79/EC, and the ISO 17511 International standard [24]. The results were within the recommended values issued for the SeronormTM Trace Elements Urine L-1 and SeronormTM Trace Elements Urine L-2. The measurement repeatability was investigated in two different urine samples, one with visible precipitates and the other one completely transparent (each one with n = 5). The relative standard deviation (RSD) was 2.3% for iodine, 1.3% for selenium (up to 6.5% for samples with low concentrations), and < 1.0% for arsenic. UCC was measured at Fürst Medical Laboratory, Oslo, Norway.
Definitions
Epidemiological criteria defined by WHO [5] for median UIC (not creatinine adjusted) was used to assess population iodine status; UIC < 20 µg/L severe iodine deficiency; UIC < 50 µg/L moderate iodine deficiency; UIC < 100 µg/L mild iodine deficiency; UIC in the range 100–199 µg/L adequate iodine intake; UIC in the range 200–299 µg/L more than adequate iodine intake; UIC > 300 µg/L excessive iodine intake. There is no established reference value for evaluating selenium or arsenic status based on urinary concentration. Thus, selenium and arsenic urinary concentrations were compared against measures of urinary selenium and arsenic in healthy populations [25, 26]. The urinary concentration of selenium range from 10 to 90 μg/L [26]. Regarding arsenic, in a European reference population (with no occupational exposure, no seafood consumption and drinking water concentration below 10 µg/L), mean concentrations of urinary inorganic arsenic and related metabolites are around 5–6 µg/L [27].
We adjusted UIC for creatinine for each participant by the following equation [28, 29]:

Analysis of thyroid function markers
We collected a non-fasting venous blood sample and measured serum thyroglobulin (Tg), serum thyroid-stimulating hormone (S-TSH), free triiodothyronine (S-fT3), free thyroxine (S-fT4) and serum anti-TPO (S-anti-TPO). S-Tg was measured in duplicate using a sandwich serum Tg enzyme-linked immunosorbent assay (ELISA) [30]. Liquicheck Tumor Marker Control (Bio-Rad Laboratories AG, Cressier, Switzerland; LOT. 24000) was used as standard. We used laboratory-specific external quality control samples and the presented data complied with the defined criteria. The limit of detection for serum Tg assay was 2.3 µg/L [30]. For S-Tg concentrations below the limit of detection (LOD) (2.3 µg/L), we used a number generator and assigned a random value between 0.1 and 2.3 µg/L. S-TSH, S-fT4 and S-fT3 were analyzed at Fürst Medical Laboratory, Oslo, Norway using Advia Centaur XPT-instruments (Siemens Healthineers, City, Country).
The reference values from Fürst medical laboratory (Oslo, Norway) were used to evaluate S-TSH, S-fT3, S-fT4 and S-Anti-TPO levels. S-TSH reference range 0.20–4.0 mU/L (> 19 years of age); S-fT3 reference range 3.5–6.5 pmol/L; S-fT4 reference range 11.0–23.0 pmol/L and S-Anti-TPO a cutoff > 100 kU/L were used. We defined thyroid dysfunction as outlined in Supplemental Table 1. No reference values are available in adults for the used Tg assay.
Statistics
IBM SPSS versions 25, 27 and 29 (IBM Corp., Armonk, NY, USA) were used for statistical analysis. Normality of the data was checked using visual evaluation of the Q–Q plots and histograms. Normally distributed data were presented as mean ± standard deviation (SD) and non-normally distributed data as median and the 25th and 75th percentiles (p25–p75) in tables. Cross-tabulation with Chi-square test was used to test differences between the dietary groups at nominal level; gender (male, female) and supplement use (yes/no). Kruskal–Wallis test with correction for multiple comparison was used to test for difference between the dietary groups, and difference in the Bonferroni post hoc test is indicated with equal superscripts. p value < 0.05 was used as significance value throughout.
Multiple linear regression analysis was used to examine the association between vegan dietary practice with Tg levels. Before performing a multivariate-adjusted analysis, univariate regression analysis was performed to examine if there was an association between the independent variable (vegan diet coded as dummy variable with pescatarian as control group) with the Tg levels (dependent). The independent variables (age, gender, smoking, supplement use, and years of dietary practice) that were significantly associated with the Tg levels in the univariate regression analysis (significance level p value < 0·005) were included in the multiple regression analysis. Two cases were identified with standardized residuals above 3. Sensitivity analysis was performed with and without the outliers. The final model was adjusted for age (years, continuous) and gender (male ref).
Results
The characteristics of the study population (n = 205) are shown in Table 1. Gender, diet duration and habitual use of iodine and selenium supplements differed between the diet groups, while characteristics as age, body mass index (BMI), educational level, smoking status, 24-h iodine supplement use and macroalgae use (habitual use), did not differ between the diet groups.
Thyroid function markers
The overall median (p25, p75) Tg in the study population was 17 (9, 30) µg/L and vegans had higher median Tg compared to pescatarians (p = 0.028) Table 2. TSH was elevated (> 4.0 mU/L) in 3% of the study participants. The prevalence of subclinical hypothyroidism (elevated TSH) did not differ between vegans, lacto-ovo vegetarians, and pescatarians (p = 0.824). None of the participants had fT4 levels below < 11.0 pmol/L. We observed a significant, but weak, correlation between Tg and TSH (p = 0.041, rs = 0.143), but no correlation between Tg and fT4 (p = 0.331, rs = − 0.068). We observed no correlation between UIC and Tg (p = 0.073), TSH (p = 0.480), fT4 (p = 0.489) and anti-TPO (p = 0.540). Anti-TPO positivity (> 100 kU/L) was found in 18% (n = 204), of which four were vegans and five lacto-ovo vegetarians.
In vegans, median Tg was significantly higher in non-supplement users compared to supplement users (p = 0.004) (Table 3).
Multiple linear regression analysis (Table 4) was used to examine the association between a diet without any iodine food sources (vegan dietary practice) and a diet including all iodine source (pescatarian dietary practice as control group) (independent variables) with Tg levels (dependent variable). Before performing a multivariate-adjusted analysis, univariate regression analysis (unadjusted) was performed to examine if there was an association between the independent variable (vegan diet coded as dummy variable with pescatarian as control group) with the Tg levels (dependent) (unadjusted). The independent variable was significantly associated with the Tg levels in the univariate regression analysis (unadjusted) (significance level p value < 0.005). The estimates were not influencing the regression model and were included in the final model. The final model was adjusted for age (years, continuous) and gender (male ref).
Urinary concentration of iodine, creatine-adjusted iodine concentration, selenium and arsenic
The median (p25, p75) UIC, creatine-adjusted UIC, selenium- and arsenic concentration in urine are presented in Table 5. The median UIC was 57 (28, 130) µg/L indicating overall inadequate iodine intake (UIC < 100 µg/L). Median creatine-adjusted UIC was 139 (63, 298) µg/day.
Vegans had lower median UIC compared to lacto-ovo vegetarians and pescatarians (p = 0.030), but when accounting for hydration (creatinine adjusted UIC), we observed no difference in UIC between the three diet groups (p = 0.270). Iodine supplement users had higher median UIC compared to non-iodine supplement users in all dietary groups [23]. For non-supplement users of iodine, vegans had lower median UIC compared to pescatarians (p = 0.002). None of the participants reported macroalgae consumption the previous 24 h. The overall median urinary concentration of arsenic was below < 15 µg/L. Vegans and lacto-ovo vegetarians had median arsenic concentrations < 15 µg/L and significantly higher concentrations (> 15 µg/L) were found in pescatarians (p < 0.001).
Discussion
A normal thyroid gland can adapt to mild iodine deficiency and maintain thyroid hormone production [31]. The physiological response involves increased blood Tg concentration and higher thyroid activity [7]. Since there are no reference values for Tg in adults, we compared differences in Tg between the dietary groups. Vegans had higher Tg concentration compared to pescatarians, indicating that a low iodine intake increase thyroid activity and the thyroid production of Tg. Vegan non-supplement users had higher Tg compared to vegans consuming an iodine-containing supplements, consistent with other studies [7, 32]. Larger epidemiological studies have shown that prolonged thyroid stimulation associated with such an adaptation leads to thyroid growth, and during follicular cell proliferation, there is a tendency to mutations leading to multifocal autonomous growth and dysfunction [31, 33]. In populations with mild and moderate iodine deficiency, such multifocal autonomous thyroid function is a common cause of hyperthyroidism [34], particularly in elderly people, and the prevalence of thyroid enlargement and nodularity is higher than in iodine sufficient populations [31, 33]. In Denmark, profound effects of even minor differences in iodine intake level on the prevalence of goiter, nodules, and hyperthyroidism have been reported [35]. However, the incidence of multinodular toxic goiter and thyrotoxicosis decreased with improved iodine intake [36].
We found a low prevalence of thyroid dysfunction in our study and fT3, fT4 and TSH were within normal ranges. In the Adventist Health Study-2, there was no association between vegan diets and hypothyroidism [37]. However, in a more recent study focusing on a subsample of the Adventist Health Study-2, Tonstad found that those with elevated TSH (> 5 mUI/L) were more likely to be women following a vegan or vegetarian diet [38]. Leung et al. conducted a study in 140 vegetarians and vegans in USA and found TSH and FT4 concentrations in the normal range [39]. Although serum TSH was generally normal in 101 British vegans, mean TSH was 47% higher than for omnivores [40]. No thyroid function abnormalities were found in Swedish and Finnish vegans [41, 42].
Median UIC in the participating vegans, lacto-ovo-vegetarians and pescatarians indicated mild- to moderate iodine deficiency. Even in those using iodine supplements, UIC indicated inadequate iodine intake. It might be explained by the fact that iodine supplements with 150 µg iodine/day might not be sufficient in a people adhering to plant-based diets, or it can be challenges related to compliance with iodine supplements. In Norway, the iodine fortification of table salt is voluntary, and the permitted level of iodine is only 5 µg/g salt thus table salt is considered a negligible iodine source in the Norwegian diet [4]. Ovo-lacto vegetarians and pescatarian have iodine sources such as milk, dairy products, and eggs (fish for the pescatarian). Vegans have few iodine sources in their diet and are dependent on iodine supplements or intake of macroalgae to maintain an adequate iodine intake.
In our study, creatine-adjusted UIC, was higher than UIC. There are two factors that may contribute to this apparent disparity: with no meat intake in the study population, the total amount of creatinine destined for urine excretion (as creatinine) per 24 h may be less than in the general population from where the adjustment equation originates, inflating the creatine-adjusted UIC [43]. Second, a high urine volume may have diluted the iodine concentration in the spot sample reducing the expected UIC [6]. The WHO cutoff value for iodine deficiency is, however, not adjusted for creatinine, therefore, we compare UIC in our study with the WHO guidelines. WHO recommend use of spot urine as an easy and cost-efficient method for assessing iodine status in population groups. However, there is large inter- and intra-individual variation in UIC caused by differences in iodine intake as well as by large variation in fluid intake [5].
The total sample median urinary selenium concentration (13 µg/L) were below the reported levels in the Canadian Health Measures Survey [reference value (RV95)] of 120 µg/L [25] and in a Belgian population (RV95 of 62 µg/L) [44]. The levels are, however, within the same range as seen in a study of blood and urine of healthy unexposed subjects living in different regions of the United Kingdom, where a reference interval from 6 to 43 µg/L was set for selenium [45]. The findings of relatively low urinary selenium in our study are also comparable to a study by Fallon [46], which found low intakes and selenium in women of fertile age adhering to strict plant-based diets. Worldwide selenium intake varies considerably, with populations in Europe having relatively low selenium intake, compared to, e.g., North America and some parts of South America and Asia where selenium content of soil is high [47]. In this study, no difference was observed in selenium concentration between the different dietary groups. In Norway, fish was recently calculated to contribute with over 20% of the total mean intake of selenium for adults (general population) [48].
The median urinary concentrations of arsenic (3 µg/L) were low compared to a previous study from Norway where median total arsenic levels in urine were 102 µg/L, ranging from 8 to 859 µg/L [49]. Median urinary concentrations of arsenic in our study were also lower compared to the European reference populations where mean concentrations of urinary inorganic arsenic and related metabolites were 5–6 µg/L [27]. Total arsenic comprise of different arsenic species, whereas inorganic arsenic and the simple methylated forms are generally present in urine [27]. The toxicity of arsenic depends on the chemical form present, with inorganic arsenic being more toxic than organic arsenic compounds, although many compounds not yet fully characterized for their toxicity. Fish and seafood generally contain high levels of total arsenic, whereas the concentration of inorganic arsenic is usually low [27]. In most seafood, arsenic is mainly present in the form of arsenobetaine, which is assumed to be of no toxicological concern and is excreted unchanged in urine [27]. According to a risk assessment conducted by European Food Safety Authority (EFSA), Norway was considered the country with highest intake of arsenic in Europe, due to the high consumption of fish [27]. In Norway, foods with high concentration of inorganic arsenic are rice and rice products, supplements based on algae, and shellfish [50]. However, cereals and cereal products, vegetables, bottled water, and coffee contribute with the highest intake because they are consumed more frequently or in higher volumes [28].
Our study adds important data on iodine nutrition and thyroid function in population groups consuming vegan, lacto-ovo vegetarian and pescatarian diets. A strength of this study was the sample size of over 200 participants, compared to previous studies in people following plant-based diets. Another strength is that we measured several indicators of thyroid function and iodine nutrition: thyroid hormones, thyroglobulin, iodine supplement, macroalgae and urinary iodine. Limitations of the study were: lack of reference values for thyroglobulin [28] and the non-randomized study design, which resulted in smaller sample size in the pescatarian groups. The study also lacked a control group, but we included pescatarian groups which include all the dietary iodine sources in Norway. However, it is still a limitation that we did not include an omnivore group, as pescatarians might differ from the general populations as they might limit the intake of dairy products, eggs, and seafood to more extent.
Conclusions
The prevalence of subclinical hypothyroidism was low and fT4 and fT3 were within the normal range for all dietary groups. Vegans had significantly increased Tg compared to pescatarians. All dietary groups had UIC indicating mild-to-moderate iodine deficiency, with lowest UIC in vegans. No differences were found between dietary groups in selenium- and arsenic concentrations. Although a low iodine intake may not have an acute effect on thyroid function, over time it may increase the risk of thyroid disorders, thus individuals with low iodine intakes are recommended to consume dietary iodine supplements.
Data Availability
The data presented in this study are available on request from the corresponding author.
References
Zimmermann BM (2011) The role of iodine in human growth and development. Semin Cell Dev Biol 22(6):645–652. https://doi.org/10.1016/j.semcdb.2011.07.009. (Epub 2011 Jul 23)
Ittermann T, Albrecht D, Arohonka P, Bilek R, de Castro JJ, Dahl L et al (2020) Standardized map of iodine status in Europe. Thyroid 30(9):1346–1354. https://doi.org/10.1089/thy.2019.0353
Groufh-Jacobsen S, Mosand LM, Bakken KS, Solvik BS, Oma I, Gjengedal ELF et al (2020) Mild to moderate iodine deficiency and inadequate iodine intake in lactating women in the inland area of Norway. Nutrients. https://doi.org/10.3390/nu12030630
Henjum S, Abel MH, Meltzer HM, Dahl L, Alexander J, Torheim LE et al (2019) Is iodine intake adequate in Norway? Tidsskr Nor Laegeforen. https://doi.org/10.4045/tidsskr.18.0319. (Er inntaket av jod i befolkningen tilstrekkelig?)
WHO (2007) Assessment of iodine deficiency disorders and monitoring their elimination—a guide for programme managers. https://www.who.int/nutrition/publications/micronutrients/iodine_deficiency/9789241595827/en/
Zimmermann MB, Andersson M (2012) Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 70(10):553–570. https://doi.org/10.1111/j.1753-4887.2012.00528.x
Ma ZF, Skeaff SA (2014) Thyroglobulin as a biomarker of iodine deficiency: a review. Thyroid 24(8):1195–1209. https://doi.org/10.1089/thy.2014.0052
Citterio CE, Targovnik HM, Arvan P (2019) The role of thyroglobulin in thyroid hormonogenesis. Nat Rev Endocrinol 15(6):323–338. https://doi.org/10.1038/s41574-019-0184-8
Neufingerl N, Eilander A (2021) Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. https://doi.org/10.3390/nu14010029
Aakre I, Tveito Evensen L, Kjellevold M, Dahl L, Henjum S, Alexander J et al (2020) Iodine status and thyroid function in a group of seaweed consumers in Norway. Nutrients. https://doi.org/10.3390/nu12113483
Aakre I, Solli DD, Markhus MW, Mæhre HK, Dahl L, Henjum S et al (2021) Commercially available kelp and seaweed products—valuable iodine source or risk of excess intake? Food Nutr Res. https://doi.org/10.29219/fnr.v65.7584
Clegg ME, Tarrado Ribes A, Reynolds R, Kliem K, Stergiadis S (2021) A comparative assessment of the nutritional composition of dairy and plant-based dairy alternatives available for sale in the UK and the implications for consumers’ dietary intakes. Food Res Int 148:110586. https://doi.org/10.1016/j.foodres.2021.110586
Bath SC, Hill S, Infante HG, Elghul S, Nezianya CJ, Rayman MP (2017) Iodine concentration of milk-alternative drinks available in the UK in comparison with cows’ milk. Br J Nutr 118(7):525–532. https://doi.org/10.1017/s0007114517002136
Winther KH, Rayman MP, Bonnema SJ, Hegedüs L (2020) Selenium in thyroid disorders—essential knowledge for clinicians. Nat Rev Endocrinol 16(3):165–176. https://doi.org/10.1038/s41574-019-0311-6
Schomburg L (2017) Plant-based diets and selenium intake and status. In: Mariotti F (ed) Vegetarian and Plant-based diets in health and disease prevention. Academic Press, Cambridge, pp 729–746
Combs GF Jr (2015) Biomarkers of selenium status. Nutrients 7(4):2209–2236. https://doi.org/10.3390/nu7042209
EFSA (2014) Scientific opinion on dietary reference values for selenium. EFSA J 12(10):3846. https://doi.org/10.2903/j.efsa.2014.3846
Phiri FP, Ander EL, Lark RM, Bailey EH, Chilima B, Gondwe J et al (2020) Urine selenium concentration is a useful biomarker for assessing population level selenium status. Environ Int 134:105218. https://doi.org/10.1016/j.envint.2019.105218
Palazzolo DL, Jansen KP (2008) The minimal arsenic concentration required to inhibit the activity of thyroid peroxidase activity in vitro. Biol Trace Elem Res 126(1–3):49–55. https://doi.org/10.1007/s12011-008-8183-y
Molin M, Ulven SM, Dahl L, Lundebye AK, Holck M, Alexander J et al (2017) Arsenic in seafood is associated with increased thyroid-stimulating hormone (TSH) in healthy volunteers—a randomized controlled trial. J Trace Elem Med Biol 44:1–7. https://doi.org/10.1016/j.jtemb.2017.05.004
Vahter M (2002) Mechanisms of arsenic biotransformation. Toxicology 181–182:211–217. https://doi.org/10.1016/s0300-483x(02)00285-8
Hughes MF (2006) Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect 114(11):1790–1796. https://doi.org/10.1289/ehp.9058
Groufh-Jacobsen S, Hess SY, Aakre I, Folven Gjengedal EL, Blandhoel Pettersen K, Henjum S (2020) Vegans, vegetarians and pescatarians are at risk of iodine deficiency in Norway. Nutrients. https://doi.org/10.3390/nu12113555
ISO (2003) In vitro diagnostic medical devices—measurement of quantities in samples of biological origin—metrological traceability of values assigned to calibrators and control materials. https://www.iso.org/standard/69984.html
Saravanabhavan G, Werry K, Walker M, Haines D, Malowany M, Khoury C (2017) Human biomonitoring reference values for metals and trace elements in blood and urine derived from the Canadian Health Measures Survey 2007–2013. Int J Hyg Environ Health 220(2):189–200. https://doi.org/10.1016/j.ijheh.2016.10.006
Hadrup N, Ravn-Haren G (2020) Acute human toxicity and mortality after selenium ingestion: a review. J Trace Elem Med Biol 58:126435. https://doi.org/10.1016/j.jtemb.2019.126435
EFSA (2009) Scientific opinion on arsenic in food. EFSA J 7(10):1351
Andersson M, Hunziker S, Fingerhut R, Zimmermann MB, Herter-Aeberli I (2020) Effectiveness of increased salt iodine concentration on iodine status: trend analysis of cross-sectional national studies in Switzerland. Eur J Nutr 59(2):581–593. https://doi.org/10.1007/s00394-019-01927-4
Kesteloot H, Joossens JV (1996) On the determinants of the creatinine clearance: a population study. J Hum Hypertens 10(4):245–249
Stinca S, Andersson M, Erhardt J, Zimmermann MB (2015) Development and validation of a new low-cost enzyme-linked immunoassay for serum and dried blood spot thyroglobulin. Thyroid 25(12):1297–1305. https://doi.org/10.1089/thy.2015.0428
Zimmermann MB, Boelaert K (2015) Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol 3(4):286–295. https://doi.org/10.1016/s2213-8587(14)70225-6
Krejbjerg A, Bjergved L, Bülow Pedersen I, Carlé A, Knudsen N, Perrild H et al (2016) Serum thyroglobulin as a biomarker of iodine deficiency in adult populations. Clin Endocrinol (Oxf) 85(3):475–482. https://doi.org/10.1111/cen.13037
Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S et al (2010) Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab 24(1):13–27. https://doi.org/10.1016/j.beem.2009.08.013
Petersen M, Knudsen N, Carlé A, Andersen S, Jørgensen T, Perrild H et al (2019) Increased incidence rate of hypothyroidism after iodine fortification in Denmark: a 20-year prospective population-based study. J Clin Endocrinol Metab 104(5):1833–1840. https://doi.org/10.1210/jc.2018-01993
Laurberg P, Jørgensen T, Perrild H, Ovesen L, Knudsen N, Pedersen IB et al (2006) The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol 155(2):219–228. https://doi.org/10.1530/eje.1.02210
Petersen M, Bülow Pedersen I, Knudsen N, Andersen S, Jørgensen T, Perrild H et al (2019) Changes in subtypes of overt thyrotoxicosis and hypothyroidism following iodine fortification. Clin Endocrinol (Oxf) 91(5):652–659. https://doi.org/10.1111/cen.14072
Tonstad S, Nathan E, Oda K, Fraser G (2013) Vegan diets and hypothyroidism. Nutrients 5(11):4642–4652. https://doi.org/10.3390/nu5114642
Tonstad S, Jaceldo-Siegl K, Messina M, Haddad E, Fraser GE (2016) The association between soya consumption and serum thyroid-stimulating hormone concentrations in the Adventist Health Study-2. Public Health Nutr 19(8):1464–1470. https://doi.org/10.1017/s1368980015002943
Leung AM, Lamar A, He X, Braverman LE, Pearce EN (2011) Iodine status and thyroid function of Boston-area vegetarians and vegans. J Clin Endocrinol Metab 96(8):E1303–E1307. https://doi.org/10.1210/jc.2011-0256
Key T, J., A, Thorogood M, Keenan J, Long A. (1992) Raised thyroid stimulating hormone associated with kelp intake in British vegan men. J Hum Nutr Diet 5(5):323–326
Rauma AL, Törmälä ML, Nenonen M, Hänninen O (1994) Iodine status in vegans consuming a living food diet. Nutr Res 14(12):1789–1795. https://doi.org/10.1016/S0271-5317(05)80715-8
Abdulla M, Andersson I, Asp NG, Berthelsen K, Birkhed D, Dencker I et al (1981) Nutrient intake and health status of vegans. Chemical analyses of diets using the duplicate portion sampling technique. Am J Clin Nutr 34(11):2464–2477. https://doi.org/10.1093/ajcn/34.11.2464
Delanghe J, De Slypere JP, De Buyzere M, Robbrecht J, Wieme R, Vermeulen A (1989) Normal reference values for creatine, creatinine, and carnitine are lower in vegetarians. Clin Chem 35(8):1802–1803. https://doi.org/10.1093/clinchem/35.8.1802
Hoet P, Jacquerye C, Deumer G, Lison D, Haufroid V (2013) Reference values and upper reference limits for 26 trace elements in the urine of adults living in Belgium. Clin Chem Lab Med 51(4):839–849. https://doi.org/10.1515/cclm-2012-0688
White MA, Sabbioni E (1998) Trace element reference values in tissues from inhabitants of the European Union. X. A study of 13 elements in blood and urine of a United Kingdom population. Sci Total Environ 216(3):253–270. https://doi.org/10.1016/s0048-9697(98)00156-9
Fallon N, Dillon SA (2020) Low intakes of iodine and selenium amongst Vegan and Vegetarian women highlight a potential nutritional vulnerability. Front Nutr 7:72. https://doi.org/10.3389/fnut.2020.00072
Rayman MP, Rayman MP (2002) The argument for increasing selenium intake. Proc Nutr Soc 61(2):203–215. https://doi.org/10.1079/pns2002153
VKM (2022) Benefit and risk assessment of fish in the Norwegian diet
Birgisdottir BE, Knutsen HK, Haugen M, Gjelstad IM, Jenssen MTS, Ellingsen DG et al (2013) Essential and toxic element concentrations in blood and urine and their associations with diet: Results from a Norwegian population study including high-consumers of seafood and game. Sci Total Environ 463–464:836–844. https://doi.org/10.1016/j.scitotenv.2013.06.078
VKM (2016) Dietary exposure to inorganic arsenic in the Norwegian population
Acknowledgements
We thank Sara Gessler for measuring serum Tg.
Funding
Open access funding provided by OsloMet - Oslo Metropolitan University. This research was funded by OsloMet.
Author information
Authors and Affiliations
Contributions
Conceptualization, SH; methodology, SH and SG-J; formal analysis, SG-J; investigation, SG-J; writing—original draft preparation, SH and SG-J; writing—review and editing, SH, SG-J, IA, ELFG, EH, MA; project administration, SH; funding acquisition, SH. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki. The study had clearance from the Regional Committee for Medical and Health Research Ethics, 2019/653/REC Southeast, and the Norwegian Center for Research Data/NSD/101332.
Informed consent
Informed consent was obtained from all the subjects involved in the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Henjum, S., Groufh-Jacobsen, S., Aakre, I. et al. Thyroid function and urinary concentrations of iodine, selenium, and arsenic in vegans, lacto-ovo vegetarians and pescatarians. Eur J Nutr 62, 3329–3338 (2023). https://doi.org/10.1007/s00394-023-03218-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03218-5