Abstract
Purpose
Changes in dietary protein intake metabolically affect kidney functions. However, knowledge on potential adverse consequences of long-term higher protein intake (HPI) for kidney health is lacking. To summarise and evaluate the available evidence for a relation between HPI and kidney diseases, an umbrella review of systematic reviews (SR) was conducted.
Methods
PubMed, Embase and Cochrane Database of SRs published until 12/2022 were searched for the respective SRs with and without meta-analyses (MA) of randomised controlled trials or cohort studies. For assessments of methodological quality and of outcome-specific certainty of evidence, a modified version of AMSTAR 2 and the NutriGrade scoring tool were used, respectively. The overall certainty of evidence was assessed according to predefined criteria.
Results
Six SRs with MA and three SRs without MA on various kidney-related outcomes were identified. Outcomes were chronic kidney disease, kidney stones and kidney function-related parameters: albuminuria, glomerular filtration rate, serum urea, urinary pH and urinary calcium excretion. Overall certainty of evidence was graded as ‘possible’ for stone risk not to be associated with HPI and albuminuria not to be elevated through HPI (above recommendations (> 0.8 g/kg body weight/day)) and graded as ‘probable’ or ‘possible’ for most other kidney function-related parameters to be physiologically increased with HPI.
Conclusion
Changes of the assessed outcomes may have reflected mostly physiological (regulatory), but not pathometabolic responses to higher protein loads. For none of the outcomes, evidence was found that HPI does specifically trigger kidney stones or diseases. However, for potential recommendations long-term data, also over decades, are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary habits are known to metabolically affect functions of numerous organs, including those of the kidney. Although single nutritional factors appear not to be directly or strongly involved in kidney function decline, long-term nutritional habits can relevantly contribute to renal impairment by modulating risk factors for chronic kidney disease (CKD), such as hypertension, hyperglycaemia, or obesity [1]. Moreover, several nutritional factors are known to play a crucial role in kidney stone formation [2]. One major nutrient, i.e. protein, has been in the focus of renal nutritional research for many decades since high-protein intake (HPI) has been observed in different studies to raise the risk of renal death, to accelerate the onset of dialysis in patients with CKD, and to increase urinary risk factors for kidney stone formation [3, 4]. However, conclusive findings on potential long-term consequences of habitually eating high-protein diets for kidney health are lacking in subjects with normal renal function [5].
Among the various outcomes related to kidney function assessment, urinary albumin (or protein) excretion and glomerular filtration rate (GFR) are the most prominent. Along with these parameters, urine pH and urinary calcium excretion, serum concentrations of urea and uric acid are also frequently examined. Nevertheless, their specificity as indicators for kidney function decline and kidney disease development has not yet been clarified in a causal context with HPI.
The evidence-based guideline for protein intake of the German Nutrition Society addresses the key question of whether the dietary intake of protein with regard to quantitative and qualitative considerations affects the development of kidney diseases in the general adult population. The current analysis focuses on the effect of HPI (generally above 0.8 g/kg body weight (BW)/day (d)) [6]) on kidney diseases and renal function-related parameters.
Methods
We conducted an umbrella review (PROSPERO: CRD42018082395) [7] following the methodology published by Kroke et al. [8]. All methodological steps were conducted independently by two authors. Any disagreements were resolved by discussion to achieve consensus.
Literature search
The systematic literature search was conducted in PubMed, Embase and Cochrane Database of Systematic Reviews for systematic reviews (SRs) published between 02/2008 and 12/2022. The date 02/2008 originates from the decision to cover a 10-year period, i.e. the initial database search was conducted in 02/2018, and the last update was made in 12/2022. The search strategies are presented in Supplementary Material S1. In addition to the database search, reference lists of included SRs were screened. Broad overall search strategies encompassing a wide range of potential renal function-related parameters, kidney diseases, and kidney stones were applied to gather all functionally or potentially pathophysiologically relevant kidney outcomes.
Literature selection
Titles and/or abstracts of retrieved studies were screened according to predefined inclusion and exclusion criteria [8] to identify potentially eligible SRs. The full texts of these records were retrieved and assessed for eligibility. It was tolerated that some of the primary studies were incorporated more than once into different SRs. The overlap of primary studies is shown in Supplementary Material S2.
SRs were included if they met the following criteria: (i) the study evaluated the association between protein intake and kidney function-related outcomes or kidney diseases; (ii) the study population was the general adult population including older adults and athletes; (iii) the study design was an SR with or without meta-analysis (MA) of prospective studies with human study participants, i.e. randomised controlled trials (RCTs), prospective cohort studies, case–cohort studies, or nested case–control studies; SRs also considering case–control studies were only included if prospective studies were predominant (> 50% of all studies); (iv) publication was written in English or German and (v) published between 02/2008 and 12/2022 [8].
Data extraction
The following relevant data from each included SR were extracted into a standardised table: first author of the SR, year of publication, study type of relevant primary studies, study period of relevant primary studies, study population of relevant primary studies, range of protein intake if provided, intervention/exposure(s) of primary studies, outcome(s) investigated by primary studies, effect estimates including 95% CI, p values, heterogeneity estimates, and subgroup analyses.
Assessment of methodological quality and outcome-specific certainty of evidence
To assess the methodological quality of included SRs, a modified version of the AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews 2) tool [9] was used (Supplementary Material S3), and the modifications are described in detail in our methodological protocol [8]. This version of AMSTAR 2 contains 14 items that evaluate the methodological quality of the SR. SRs were rated on a scale from high quality to critically low quality according to the existence of critical and non-critical methodological weaknesses. SRs graded as ‘critically low’ by AMSTAR 2 were excluded from the rating of the overall certainty of evidence.
The outcome-specific certainty of evidence of included SRs with and without MA was assessed using the NutriGrade scoring tool [10] (Supplementary Material S4). NutriGrade aims to assess the certainty of evidence of an association or effect between different dietary factors and outcomes, taking into account nutrition research-specific requirements not considered by other tools. The NutriGrade scoring tool utilises a numerical scoring system and comprises seven items for SRs with MA of RCTs and eight items for MA of cohort studies. Based on the scoring system, four categories rate the potential outcome-specific certainty of evidence: ‘high’, ‘moderate’, ‘low’ and ‘very low’. The NutriGrade scoring tool was modified for the assessment of SRs without MA [8] (Supplementary Material S5). We adjusted the items related to MA: (i) precision: the confidence intervals were deleted, (ii) heterogeneity: this item was reduced to the question about consistency of the results, (iii) publication bias: this item was deleted, (iv) effect size: the RR/HR were deleted and (v) dose–response: this item was deleted. For SRs reporting more than one relevant outcome, each outcome was assessed separately.
Definition of the outcomes
Throughout the SRs included in this umbrella review and the underlying primary studies, the definitions of outcomes were frequently not consistent. The following outcome specifications were generally used.
Albumin excretion was either reported as albumin excretion per 24 h (24 h) or per minute, or as albumin excretion/liter quantified in 24-h urine samples, or as albumin/creatinine ratio in spot samples. Some studies used the classification microalbuminuria (30 mg–300 mg per 24 h or per mg creatinine) as an outcome, where excretion levels > 300 mg represent macroalbuminuria.
GFR, in addition to albumin excretion, also belongs to the most important indicators regularly determined to assess kidney function. Of those primary studies examining GFR, only a few used direct measurement methods to determine this outcome. Most studies applied one of several published estimating equations either based on a serum or a plasma concentration measurement of creatinine or cystatin C. Equations estimating GFR (eGFR), most frequently used, were Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), Modification of Diet in Renal Disease (MDRD), or Cockroft Gault (CG). Measured or estimated GFR (eGFR) was in general reported as “mL/min per 1.73 m2”. A GFR below 60 ml/min/1.73 m2 usually marks a relevant, still mild to moderate (if not lower than 45 ml/min/1.73 m2), decline in kidney function [11] and this cutoff (< 60 mL/min/1.73 m2) was used in respective primary studies to exclude subjects with an insufficient kidney health. Some studies simply reported serum creatinine concentrations without applying one of the GFR estimation formulas.
CKD, when used as an outcome, was assessed as incident CKD applying the eGFR stage < 60 mL/min per 1.73 m2 as criterion.
Kidney stones, used as the assessment outcome in two SRs, were mostly based on self-reported diagnoses. In general, calcium oxalate is the most common stone type, followed by carbonate apatite, uric acid, struvite, brushite and cystine [12].
Urinary calcium excretion, if elevated, is a major and common risk factor for both calcium oxalate and calcium phosphate stone formation [13, 14]. Urinary calcium excretion was measured in 24-h urine samples and reported as excretion rate per 24 h.
Urinary pH is an independent risk factor for the formation of various types of kidney stones and is regarded as an indicator of renal function. Urinary pH is usually determined in 24-h urines, applying pH meters.
Urea and uric acid blood concentrations are protein intake-related markers of renal elimination function for water-soluble, nitrogen-containing metabolic end products. These outcomes were analysed in serum samples, using standard clinical assays, in several primary studies.
Rating the overall certainty of the evidence
The overall certainty of the evidence was assessed according to the framework outlined in the methodological protocol [8] and in Table 1. For this publication, two authors (TR, RS) made suggestions for rating the overall certainty of evidence. This rating was double-checked by a staff member of the German Nutrition Society (NK) and thereafter reviewed by all co-authors. The final ratings of the overall certainty of evidence was approved by all authors. In an amendment step, rating of the overall certainty of evidence was complemented by two authors (TR and RS) with a specific evaluation on whether the outcome may unequivocally and without bias mirror a health-relevant kidney function change and not only a normal physiological adaptation to an altered protein load. The presence of unambiguous pathometabolic consequences of HPI for kidney health was highly doubted if the changes of the respective outcome either (i) reflect physiological adaptation to a higher protein intake level or (ii) (may) represent physiological consequences of literature-known, but not-considered factors co-varying with the protein amount ingested.
Results
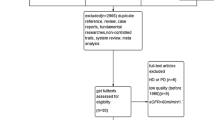
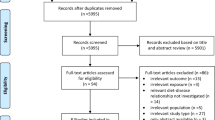
Of the 7486 publications initially identified, 9 SRs remained for analysis: 6 SRs with MA and 3 SRs without MA [15,16,17,18,19,20,21,22,23]. The study selection process is outlined in Fig. 1. A list of the excluded SRs after full-text screening, including justifications for exclusion, is provided in Supplementary Material S6. None of the SRs was excluded due to ‘critically low’ rating by AMSTAR 2.
Characteristics of the included systematic reviews
Table 2 shows the characteristics of the SRs. Three of them were SRs with MA of RCTs [16, 18, 19], three were SRs with MA of cohort studies [20,21,22] and three were SRs without MA of RCTs or cohort studies [15, 17, 23]. One SR with MA conducted a dose–response analysis [16].
The SRs investigated kidney stone formation [17, 20], CKD [21], and kidney function-related parameters, i.e. GFR [15,16,17,18, 21, 23], urinary albumin excretion [15, 18, 23], urinary calcium excretion [18], urinary pH [15, 18], serum urea [18], serum uric acid [15, 18], and simply serum creatinine, not used for GFR calculation [19, 23].
Seven SRs with or without MA of RCTs or cohort studies investigated the effect or association of total protein intake on or with kidney diseases and selected kidney function-related parameters [15,16,17,18,19, 21, 23]. Two SRs with MA examined the effect of animal protein intake on incident kidney stone formation and one SR without MA investigated the association of animal protein intake on selected kidney function-related parameters [20, 22]. One SR also examined potential differences between animal protein intake and plant protein intake [15]. The intervention duration of the primary studies was 4 days to 24 months for RCTs and 6 to 26 years for cohort studies. Participants were generally healthy, male or female, and aged ≥ 18 years. Some of the primary studies also included participants with metabolic risk factors (overweight, obesity, hypertension, hypercholesterolaemia, or diabetes mellitus type 2) [15,16,17,18,19, 21] or with (moderate) physical function limitations [23].
Type and range of protein intake
The protein intake ranged from 1.0 to 3.3 g/kg BW/d (high-protein groups) vs. 0.3 to 2.6 g/kg BW/d (control groups) or from 12.5 to 40 energy percentage (En%) (high-protein groups) vs. 5.4 to 24 En% (control groups) or from 123 to 150 g/d (high-protein groups) vs. 46 to 75 g/d (control groups) for all included RCTs investigating the association between protein intake and the outcomes.
No information was provided for the protein intake of included cohort studies in the four SRs [17, 20,21,22]. One primary study in the SR from Devries et al. reported only ‘unlimited protein consumption’ [16].
Methodological quality and outcome-specific certainty of the evidence
For each included SR, overall scores of AMSTAR 2 and NutriGrade are summarised in Table 2. Supplementary Materials S7 and S8 provide a more detailed description showing the assessments of each individual item. Methodological quality as assessed with AMSTAR 2 was rated ‘high’ for three SRs [19,20,21], moderate for five SRs [16,17,18, 22, 23], and ‘low’ for one SR [15].
The outcome-specific certainty of evidence as assessed with NutriGrade was moderate for increases in GFR (standardised mean difference (SMD): 0.19; 95% CI 0.07, 0.31; I2 = 0% [16], mean difference (MD): 7.18 ml/min/1.73 m2; 95% CI 4.45, 9.91; I2 = 52%) [18], urinary calcium excretion (MD: 25.43 mg/24 h; 95% CI 13.62, 37.24; I2 = 90%) [18], and serum urea (MD: 1.75 mmol/l; 95% CI 1.13, 2.37; I2 = 88%) [18]; ‘low’ for constancy of urinary albumin [18], serum uric acid [18], and urinary pH [18]; and also ‘low’ in two other SRs for GFR [17] and serum creatinine constancies [23]. A ‘very low’ outcome-specific certainty of evidence was additionally seen for constancy of GFR [21], as well as for increases in GFR (8 out of 13 RCTs were erroneously reported by van Elswyk et al. [15] instead of 8 out of 11 RCTs that actually showed significantly higher GFR in response to increased protein intake (see Discussion)) [15], and increases in serum uric acid (3 of 4 RCTs reported elevated serum uric acid in response to increased protein intake) [15] and serum creatinine (SMD: 6.14; 95% CI 2.49, 9.79; I2 = 0%) [19]. In the latter SR of Santesso et al. [19], only two out of six identified primary studies on serum creatinine were taken into account. None of these kidney function-related outcomes except the urine pH showed any substantial inverse relationship with the amounts of protein ingested. Based on one SR with MA of cohort studies, a NutriGrade rating of ‘low’ was obtained for a 10% increase (RR: 1.1; 95% CI, 1.02–1.19) in the risk of incident stone formation with the exposition variable animal protein intake [20], while two other SRs (one without and one with MA) found the risk inconclusive [17] or found no effect of animal protein on the risk of incident stone formation [22].
Rating of the overall certainty of the evidence
Using the criteria outlined in Table 1, the overall certainty of evidence was rated as ‘possible’ for albuminuria to be not elevated and ‘possible’ for urinary pH to be not reduced by HPI. It was also rated as ‘possible’ for GFR, and as ‘probable’ for urinary calcium excretion and serum urea each to be physiologically (regulatorily) and not per se pathophysiologically elevated (for further details see “Discussion”). The rating of the overall certainty of evidence was ‘insufficient’ for the relationship between protein intake and serum uric acid, but it was ‘possible’ for the absence of an association between the exposition variable animal protein intake and the risk of incident kidney stone formation.
Discussion
This umbrella review, including 6 SRs with MA and 3 SRs without MA, examined the implications of HPI for kidney health. Key findings are that for daily protein ingestion above dietary recommendations, no convincing evidence could be ascertained for kidney function decline relevant relationships with urinary albumin excretion, renal GFR, and kidney stone risk. Also for the further assessed renal-related outcomes, none of the gradings of the overall certainty of evidence led to an assessment as ‘possible’ or ‘probable’ for detrimental HPI influences on kidney function.
According to the criteria given in Table 1, for the risk marker of CKD albumin excretion [11], the overall certainty of evidence was graded as ‘possible’ to be not elevated through HPI in both young and elderly healthy adults. Furthermore, overall certainty of evidence was graded as ‘possible’ for GFR and ‘probable’ for urinary calcium excretion, as well as for serum urea, to be physiologically (regulatorily) increased with HPI. It is noteworthy that this grading as ‘possible’ for GFR was partly due to a downgrading effect through the only SR [15] with an overall low methodological quality (AMSTAR 2) and a substantial miscategorisation of the outcome GFR [15]. Instead of 8 out of 13 RCTs with GFR determinations (< 2/3), as reported by the authors, actually 8 out of 11 RCTs with GFR determinations (> 2/3) showed higher GFRs with HPI [15], thus rather allowing an assessment of the overall certainty of evidence as ‘almost probable’ and not just ‘possible’.
As outlined below, the elevations of most of the outcomes along with HPI have to be interpreted cautiously, i.e. mostly as physiological regulatory responses and not as pathophysiological increases. However, it should be considered that an elevated urinary calcium excretion may represent a risk factor for calcium stone formation. Nevertheless, for kidney stone disease, ‘possible’ evidence was derived for an absence of an association with higher animal protein intake [17, 20].
As HPI has been associated with metabolic changes that can exhibit a risk for kidney stone formation in healthy individuals [4, 24], this issue is addressed in the following along with further specific comments on the examined outcomes. Physiological background explanations are provided for a far-reaching inappropriateness of urinary calcium excretion, urinary pH, serum urea, serum uric acid, and even of the important kidney function parameter GFR (or serum creatinine) as unbiased renal health outcomes for examinations in (mostly) healthy populations if no specific adjustments are conducted. Accordingly, the suitability of these parameters to unbiasedly reveal pathophysiologically relevant influences of HPI on kidney health will be critically appraised.
Albumin excretion
The current NutriGrade ratings of ‘low’ for the finding that the diagnostically important kidney parameter urinary albumin excretion and dietary protein intake are unrelated, definitely prompting that this potential absence of an albuminuria-elevating effect through HPI needs to be further examined and particularly studied for observation periods longer than 2 years.
Kidney stones
The prevalence of urinary stone disease in the general population has been reported to range between 4.7% and 8.8% [25, 26]. Kidney stone formation is associated with an elevated risk of chronic and end-stage kidney disease, probably due to kidney injury from obstructive nephropathy [27, 28]. An HPI may promote the risk of stone formation by providing an acid load that could lead to several metabolic changes, including decreases in urinary pH and citrate excretion, and increases in urinary calcium and uric acid excretion [24, 29,30,31]. A higher dietary net acid load, estimated by animal protein-to-potassium ratio or net acid excretion (NAE), was associated with a higher risk of kidney stone formation in large observational studies [32]. These data suggest that the proportion of the consumed amount of alkalising fruits and vegetables compared to the total amount of ingested protein could modify the risk of HPI for kidney stone formation. It is beyond controversy that fruits and vegetables have a marked alkalising potential and can in such a way relevantly neutralise the proton load, metabolically generated from ingested protein [33, 34]. High dietary acidity, resulting in lower urine pH, is a risk factor for several kidney stone types, particularly for the most common, i.e. calcium oxalate stones. The higher the urine pH, the higher is the stone-inhibiting citrate excretion and calcium-binding capacity and the lower is the urinary calcium excretion [35].
In conclusion, a number of protein intake-related, metabolic, and idiopathic risk factors and confounders, such as low or high urine pH, hypercalciuria, hypocitraturia, hyperuricosuria, hyperoxaluria and further dietary/environmental risk factors, such as high sodium chloride intake and low urine volume [36, 37], all complicate a straight examination of ‘the inherent impact of protein’ on stone formation. However, most of these risk factors can at least partly be avoided or reduced by the respective changes in dietary habits, e.g. by increasing the habitual intake of metabolically alkalising fruits and vegetables [32].
GFR
Increases in GFR frequently occur during the first years after onset of diabetes mellitus type 1 or 2 [38]. This phenomenon is termed glomerular hyperfiltration. With advancing duration of the disorder, hyperfiltration regresses again and frequently turns into a pathophysiological decline of GFR. Increased body fatness and obesity also lead to elevations in GFR, independent of hyperglycaemia and other metabolic and hormonal signals also present in diabetes.
Another major stimulus of GFR is protein intake. GFR increases, lasting for several hours, occur after protein-rich meals [39], implying that if HPI and blood sampling are temporally relatively far apart (overnight fasting or even longer), GFR increases can, but may not necessarily, be any longer detectable with the use of mere serum measurement-based estimates (eGFR), although they would be observable by 24-h urine-based GFR measures. This is one of a number of explanations why in all MAs and SRs that included the outcome GFR, at least some primary studies were present which did not find GFR increases following increased protein ingestion by healthy subjects. In principle, elevations in GFR are basic, physiologically adaptive mechanisms induced by HPI in case of normal kidney function state [39,40,41,42,43].
In line herewith, none of the SRs of this umbrella review found clear indications for a GFR reduction due to HPI. Accordingly, one could classify GFR increases or at least GFR stability as a very probable consequence of raises in protein intake above dietary recommendations in the healthy state, despite the fact that the formal use of the modified grading system [8] only resulted in a grading of ‘possible’ for the overall certainty of evidence for the absence of GFR reductions.
Among others, not only younger age (until around 35 years) [44] and HPI [39,40,41,42,43], but also increases in BW [41], BMI or fat mass [45, 46], insulin resistance [47, 48], insulin secretion [49], and sodium chloride intake [45, 50, 51] all have a GFR-elevating potential. Accordingly, the examination of a potential kidney function decline by using GFR reduction as a marker or an outcome in initially metabolically healthy subjects appears – at first glance – not ideal for an exposure that by itself biologically raises GFR. GFR changes, however, should be studied in the future (as far as possible bias free) as a major outcome for the assessment of gradual kidney function decline over periods of more than 5–10 years by more appropriately controlling relevant confounders.
Urinary calcium excretion
Various dietary factors affect urinary calcium excretion, particularly the intakes of calcium, protein, and sodium chloride. In healthy subjects, intestinal calcium absorption is approximately 25% [52]. However, intestinal hyperabsorption of calcium is frequently diagnosed in stone formers [53]. Higher dietary protein intakes are consistently reported to increase urinary calcium excretion [18, 54, 55], in part due to the increased GFR [56] (see above). Apart from calcium and protein intake, urinary calcium excretion is also related to urinary NAE and acidotic stimuli [57]. While the administration of 1.5 g/d L-methionine did not significantly raise urinary calcium excretion in healthy subjects [58], the supplementation of 3 g/d L-methionine resulted in a significant increase in urinary calcium excretion by about 1 mmol/d (40 mg/d) in parallel with a rise in urinary NAE of 40 mEq/d [59]. Accordingly, without specific adjustments for the aforementioned confounding influences, the utilisation of urinary calcium excretion as an important urolithiasis-related renal health outcome appears to be less useful.
Urinary pH
Urinary pH marks the small amount of free hydrogen ions (H+) not buffered by ammonia and titratable acid (i.e. mostly phosphate) and reflects, to some degree, the overall excess of H+ that is renally secreted. The overall, i.e. the buffered amount of H+ daily eliminated by the kidney is quantified as NAE [60,61,62]. Although 24-h urine pH and NAE/d usually show good correlations [34, 63], a variation in renal buffer supply can markedly change the usual pH–NAE relationship. One major confounder in this regard is protein intake itself. The higher the protein intake, the higher is the kidney´s capacity to excrete surplus H+ [64, 65]. Accordingly, if protein intake increases and NAE is constant (through higher alkali intake), the ammonia buffer is much more easily renally provided. This means that a lower free proton stress (a lower H+ signalling) is required to increase buffer provision, i.e. ammoniagenesis. Correspondingly, in subjects without kidney disease, urine pH will be higher with HPI for every given acid load, i.e. for a constant potential renal acid load (PRAL) or a constant NAE. Thus, 24-h urine pH can only be used as a marker for kidney function change if measurements of renal 24-h NAE or PRAL are concurrently performed and appropriately adjusted for [66]. Even with HPI of around 80 to 100 g/d, mean 24-h urine pH can be kept at ≥ 6 through moderate alkali equivalent ingestion [33, 64]. Besides this, HPI with a higher NAE, higher age (> 50 years) [67], higher BMI or body fat [66, 68, 69], and other features of the metabolic syndrome including insulin resistance [68, 70] each contribute to urine pH reductions. Thus, the NutriGrade rating of ‘low’ obtained for a potential constancy of the urine pH along with rises in protein intake [18] suggests that HPI does not necessarily increase renal “free proton stress”. However, the examination of urinary free protons at least in combination with (reliable markers of) protein intake and the related net acid load can be a valuable tool to assess renal acid excretion function as well as stone formation risk [66, 71].
Serum urea and serum uric acid
Increases in urea, mostly within the normal physiological range, have been reported in almost all primary studies after protein intake was raised. Since elevation of serum urea above the upper limit of the normal range primarily depends on functional GFR reduction [72] and further confounders like hydration status, circulating urea rather represents an insensitive indicator of kidney function [72]. Next, serum uric acid shows varying interdependences with protein ingestion [73], purine intake, hydration status [74], and GFR [75], as well as with metabolic syndrome [76] and diabetes mellitus type 2 [77]. Thus, irrespective of the rating of the overall certainty of evidence as ‘probable’ or ‘insufficient’ for effects due to increases in protein intake, the utilisation of serum urea and uric acid, respectively, for the assessment of potential influences of HPI on kidney health appears to be a less specific approach.
Strengths and limitations
A strength of this umbrella review is that six of the included hitherto published SRs on the relevance of HPI for kidney health comprise, either exclusively or primarily, RCTs. A further strength is that we critically examined in more detail the suitability, as well as relevant physiological confounders, of those kidney parameters commonly used to investigate kidney health. However, several limitations have to be taken into account when assessing the findings of the SRs included in this umbrella review. We applied NutriGrade instead of the GRADE approach (Grading of Recommendations, Assessment, Development and Evaluation) because an important novelty of NutriGrade (published in 2016) was the modified classification for MA of RCTs and cohort studies compared with the traditional GRADE approach (initially classifying RCTs with an initial high score and cohort studies with a low score) [78]. We are aware that in the meantime, the GRADE approach was amended (adjustments published in 2019, but after the guideline methodology was established in 2017) in a way that cohort studies can now also be assigned an initially high score, when risk of bias tools such as ROBINS-I are used [79]. The intervention duration of the primary RCTs and also the protein intake levels varied considerably with ranges from 1 week to 2 years, and intakes from 12.5 to 40 En% solely in the high-protein groups, respectively. Although dietary protein sources have been provided in most primary studies, more specific statements regarding the relevance of animal vs. plant vs. dairy protein could not be drawn, particularly due to an insufficient number of corresponding specific data analyses. Furthermore, the substantial degree of heterogeneity, present for the different outcomes, could not be further assessed. Further important limitations of the current umbrella review are that (i) major primary studies, not included in the SRs or MAs, remained unconsidered and (ii) that, of the nine SRs that could be included, only 3 examined the most diagnostically conclusive outcome variable, i.e. albuminuria as well as kidney stones. The various other kidney function-related outcomes that were examined, however, showed clear weaknesses regarding a specific, i.e. an unconfounded assessment of possible kidney function impairments. Their increases (or potential urinary pH reductions) along with HPI are biologically plausible, but without direct specific pathophysiological relevance.
Conclusion
For none of the outcomes was a ‘convincing’ certainty of evidence found for detrimental effects of HPI with regard to the development of kidney diseases. However, most of the included studies were of rather short-term duration, so that a possible long-term risk over decades cannot be assessed at present. Although the overall certainty of evidence has been rated as ‘probable’ for an increase in urinary calcium excretion, a risk factor for calcium stone formation, the rating of the overall certainty of evidence revealed no relationship between protein intake and the risk of incident nephrolithiasis. Detailed future research is required into whether albumin excretion actually does not increase and GFR does not fall through protein intake levels exceeding the dietary recommendation of 0.8 g/kg BW/d over periods of more than 2 years and after decades in older age. Such long-term confirmatory studies, adequately controlled for the specified confounders, are necessary before changing or adapting statements on higher protein intake levels as being “quasi safe” or recommendable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Abbreviations
- AMSTAR 2:
-
A measurement tool to assess systematic reviews 2
- BW:
-
Body weight
- d:
-
Day
- En%:
-
Energy percentage
- CKD:
-
Chronic kidney disease
- GFR:
-
Glomerular filtration rate
- HPI:
-
Higher protein intake
- MA:
-
Meta-analysis/meta-analyses
- MD:
-
Mean difference
- NAE:
-
Net acid excretion
- RCT:
-
Randomised controlled trial
- SMD:
-
Standardised mean difference
- SR:
-
Systematic review(s)
References
Ajjarapu AS, Hinkle SN, Li M et al (2019) Dietary patterns and renal health outcomes in the general population: a review focusing on prospective studies. Nutrients 11:1877. https://doi.org/10.3390/nu11081877
Siener R (2021) Nutrition and kidney stone disease. Nutrients 13:1917. https://doi.org/10.3390/nu13061917
Sabatino A, Regolisti G, Gandolfini I et al (2017) Diet and enteral nutrition in patients with chronic kidney disease not on dialysis: a review focusing on fat, fiber and protein intake. J Nephrol 30:743–754. https://doi.org/10.1007/s40620-017-0435-5
Kok DJ, Iestra JA, Doorenbos CJ et al (1990) The effects of dietary excesses in animal protein and in sodium on the composition and the crystallization kinetics of calcium oxalate monohydrate in urines of healthy men. J Clin Endocrinol Metab 71:861–867. https://doi.org/10.1210/jcem-71-4-861
Kamper A-L, Strandgaard S (2017) Long-term effects of high-pProtein diets on renal function. Annu Rev Nutr 37:347–369. https://doi.org/10.1146/annurev-nutr-071714-034426
Richter M, Baerlocher K, Bauer JM et al (2019) Revised Reference Values for the intake of Protein. Ann Nutr Metab 74:242–250. https://doi.org/10.1159/000499374
National Institute for Health Research PROSPERO. International prospective register of systematic reviews. https://www.crd.york.ac.uk/prospero/. Accessed 08 Jun 2020
Kroke A, Schmidt A, Amini AM et al (2022) Dietary protein intake and health-related outcomes: a methodological protocol for the evidence evaluation and the outline of an evidence to decision framework underlying the evidence-based guideline of the German Nutrition Society. Eur J Nutr 61:2091–2101. https://doi.org/10.1007/s00394-021-02789-5
Shea BJ, Reeves BC, Wells G et al. (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008. https://doi.org/10.1136/bmj.j4008
Schwingshackl L, Knüppel S, Schwedhelm C et al (2016) Perspective: NutriGrade: A scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr 7:994–1004. https://doi.org/10.3945/an.116.013052
Intermountain Healthcare (2018) Management of chronic kidney disease (CKD). https://intermountainhealthcare.org/ckr-ext/Dcmnt?ncid=521395847. Accessed 19 Jul 2022
Siener R, Herwig H, Rüdy J et al (2022) Urinary stone composition in Germany: results from 45,783 stone analyses. World J Urol 40:1813–1820. https://doi.org/10.1007/s00345-022-04060-w
Siener R, Netzer L, Hesse A (2013) Determinants of brushite stone formation: a case-control study. PLoS ONE 8:e78996. https://doi.org/10.1371/journal.pone.0078996
Siener R, Schade N, Nicolay C et al (2005) The efficacy of dietary intervention on urinary risk factors for stone formation in recurrent calcium oxalate stone patients. J Urol 173:1601–1605. https://doi.org/10.1097/01.ju.0000154626.16349.d3
van Elswyk ME, Weatherford CA, McNeill SH (2018) A systematic review of renal health in healthy individuals associated with protein intake above the US Recommended Daily Allowance in randomized controlled trials and observational studies. Adv Nutr 9:404–418. https://doi.org/10.1093/advances/nmy026
Devries MC, Sithamparapillai A, Brimble KS et al (2018) Changes in kidney function do not differ between healthy adults consuming higher- compared with lower- or normal-protein diets: a systematic review and meta-analysis. J Nutr 148:1760–1775. https://doi.org/10.1093/jn/nxy197
Pedersen AN, Kondrup J, Børsheim E (2013) Health effects of protein intake in healthy adults: a systematic literature review. Food Nutr Res. https://doi.org/10.3402/fnr.v57i0.21245
Schwingshackl L, Hoffmann G (2014) Comparison of high vs. normal/low protein diets on renal function in subjects without chronic kidney disease: a systematic review and meta-analysis. PLoS ONE 9:e97656. https://doi.org/10.1371/journal.pone.0097656
Santesso N, Akl EA, Bianchi M et al (2012) Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur J Clin Nutr 66:780–788. https://doi.org/10.1038/ejcn.2012.37
Lin B-B, Lin M-E, Huang R-H et al (2020) Dietary and lifestyle factors for primary prevention of nephrolithiasis: a systematic review and meta-analysis. BMC Nephrol 21:267. https://doi.org/10.1186/s12882-020-01925-3
Kelly JT, Su G, Zhang La et al (2021) Modifiable lifestyle factors for primary prevention of CKD: a systematic review and meta-analysis. J Am Soc Nephrol 32:239–253. https://doi.org/10.1681/ASN.2020030384
Asoudeh F, Talebi S, Jayedi A et al (2022) Associations of total protein or animal protein intake and animal protein sources with risk of kidney stones: a systematic review and dose-response meta-analysis. Adv Nutr. https://doi.org/10.1093/advances/nmac013
Hengeveld LM, de Goede J, Afman LA et al (2021) Health effects of increasing protein intake above the current population reference intake in older adults: a systematic review of the Health Council of the Netherlands. Adv Nutr. https://doi.org/10.1093/advances/nmab140
Reddy ST, Wang C-Y, Sakhaee K et al (2002) Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis 40:265–274. https://doi.org/10.1053/ajkd.2002.34504
Hesse A, Brändle E, Wilbert D et al (2003) Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol 44:709–713. https://doi.org/10.1016/S0302-2838(03)00415-9
Scales CD, Smith AC, Hanley JM et al (2012) Prevalence of kidney stones in the United States. Eur Urol 62:160–165. https://doi.org/10.1016/j.eururo.2012.03.052
Shoag J, Halpern J, Goldfarb DS et al (2014) Risk of chronic and end stage kidney disease in patients with nephrolithiasis. J Urol 192:1440–1445. https://doi.org/10.1016/j.juro.2014.05.117
Dhondup T, Kittanamongkolchai W, Vaughan LE et al (2018) Risk of ESRD and mortality in kidney and bladder stone formers. Am J Kidney Dis 72:790–797. https://doi.org/10.1053/j.ajkd.2018.06.012
Gambaro G, Croppi E, Coe F et al (2016) Metabolic diagnosis and medical prevention of calcium nephrolithiasis and its systemic manifestations: a consensus statement. J Nephrol 29:715–734. https://doi.org/10.1007/s40620-016-0329-y
Siener R, Hesse A (2002) The effect of different diets on urine composition and the risk of calcium oxalate crystallisation in healthy subjects. Eur Urol 42:289–296. https://doi.org/10.1016/S0302-2838(02)00316-0
Siener R, Hesse A (2003) The effect of a vegetarian and different omnivorous diets on urinary risk factors for uric acid stone formation. Eur J Nutr 42:332–337. https://doi.org/10.1007/s00394-003-0428-0
Ferraro PM, Mandel EI, Curhan GC et al (2016) Dietary protein and potassium, diet-dependent net acid load, and risk of incident kidney stones. Clin J Am Soc Nephrol 11:1834–1844. https://doi.org/10.2215/CJN.01520216
Remer T, Manz F (1994) Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr 59:1356–1361. https://doi.org/10.1093/ajcn/59.6.1356
Remer T, Manz F (1995) Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 95:791–797. https://doi.org/10.1016/S0002-8223(95)00219-7
Moe OW, Huang C-L (2006) Hypercalciuria from acid load: renal mechanisms. J Nephrol 19. Suppl 9:S53–S61
Pak CYC (2008) Pharmacotherapy of kidney stones. Expert Opin Pharmacother 9:1509–1518. https://doi.org/10.1517/14656566.9.9.1509
Hesse A, Tiselius H-G, Siener R et al. (2009) Urinary stones: diagnosis, treatment, and prevention of recurrence, 3. überarbeitete und ergänzte Auflage. Karger
Tonneijck L, Muskiet MHA, Smits MM et al (2017) Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 28:1023–1039. https://doi.org/10.1681/ASN.2016060666
Friedlander G, Blanchet F, Amiel C (1989) Renal functional reserve. Toxicol Lett 46:227–235. https://doi.org/10.1016/0378-4274(89)90131-8
Hegsted M, Linkswiler HM (1981) Long-term effects of level of protein intake on calcium metabolism in young adult women. J Nutr 111:244–251. https://doi.org/10.1093/jn/111.2.244
Brouhard BH, LaGrone LF, Richards GE et al (1987) Somatostatin limits rise in glomerular filtration rate after a protein meal. J Pediatr 110:729–734. https://doi.org/10.1016/S0022-3476(87)80011-2
Premen AJ (1988) Potential mechanisms mediating postprandial renal hyperemia and hyperfiltration. FASEB j 2:131–137. https://doi.org/10.1096/fasebj.2.2.3277887
Wagner EA, Falciglia GA, Amlal H et al (2007) Short-term exposure to a high-protein diet differentially affects glomerular filtration rate but not acid-base balance in older compared to younger adults. J Am Diet Assoc 107:1404–1408. https://doi.org/10.1016/j.jada.2007.05.003
Fenton A, Montgomery E, Nightingale P et al (2018) Glomerular filtration rate: new age- and gender- specific reference ranges and thresholds for living kidney donation. BMC Nephrol 19:336. https://doi.org/10.1186/s12882-018-1126-8
Ogna A, Forni Ogna V, Bochud M et al (2016) Association between obesity and glomerular hyperfiltration: the confounding effect of smoking and sodium and protein intakes. Eur J Nutr 55:1089–1097. https://doi.org/10.1007/s00394-015-0923-0
Chagnac A, Herman M, Zingerman B et al (2008) Obesity-induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant 23:3946–3952. https://doi.org/10.1093/ndt/gfn379
Li Z, Woollard JR, Wang S et al (2011) Increased glomerular filtration rate in early metabolic syndrome is associated with renal adiposity and microvascular proliferation. Am J Physiol Renal Physiol 301:F1078–F1087. https://doi.org/10.1152/ajprenal.00333.2011
Dengel DR, Goldberg AP, Mayuga RS et al (1996) Insulin resistance, elevated glomerular filtration fraction, and renal injury. Hypertension 28:127–132. https://doi.org/10.1161/01.hyp.28.1.127
ter Maaten JC, Bakker SJ, Serné EH et al (1999) Insulin’s acute effects on glomerular filtration rate correlate with insulin sensitivity whereas insulin’s acute effects on proximal tubular sodium reabsorption correlation with salt sensitivity in normal subjects. Nephrol Dial Transplant 14:2357–2363. https://doi.org/10.1093/ndt/14.10.2357
Wiggins WS, Manry CH, Lyons RH et al (1951) The effect of salt loading and salt depletion on renal function and electrolyte excretion in man. Circulation 3:275–281. https://doi.org/10.1161/01.cir.3.2.275
Krikken JA, Lely AT, Bakker SJL et al (2007) The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney Int 71:260–265. https://doi.org/10.1038/sj.ki.5002011
Couzy F, Kastenmayer P, Vigo M et al (1995) Calcium bioavailability from a calcium- and sulfate-rich mineral water, compared with milk, in young adult women. Am J Clin Nutr 62:1239–1244. https://doi.org/10.1093/ajcn/62.6.1239
Pak CY, Britton F, Peterson R et al (1980) Ambulatory evaluation of nephrolithiasis. Am J Med 69:19–30. https://doi.org/10.1016/0002-9343(80)90495-7
Bengoa JM, Sitrin MD, Wood RJ et al (1983) Amino acid-induced hypercalciuria in patients on total parenteral nutrition. Am J Clin Nutr 38:264–269. https://doi.org/10.1093/ajcn/38.2.264
Shams-White MM, Chung M, Du M et al (2017) Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am J Clin Nutr 105:1528–1543. https://doi.org/10.3945/ajcn.116.145110
Greger JL (1988) Effect of variations in dietary protein, electrolytes, and vitamin D on calcium and zinc metabolism. In: Bodwell CE, Erdman JW (eds) Nutrient interactions: Proceedings of the eleventh annual basic symposium, sponsored by the Institute of Food Technologists and the International Union of Food Science and Technology, which was held in Las Vegas, Nevada, June 15–16, 1988. Dekker, New York, pp 205–208
Alexander RT, Cordat E, Chambrey R et al (2016) Acidosis and urinary calcium excretion: insights from genetic disorders. J Am Soc Nephrol 27:3511–3520. https://doi.org/10.1681/ASN.2016030305
Siener R, Struwe F, Hesse A (2016) Effect of L-methionine on the risk of phosphate stone formation. Urology 98:39–43. https://doi.org/10.1016/j.urology.2016.08.007
Remer T (2000) Influence of diet on acid-base balance. Semin Dial 13:221–226. https://doi.org/10.1046/j.1525-139x.2000.00062.x
Frassetto LA, Lanham-New SA, Macdonald HM et al (2007) Standardizing terminology for estimating the diet-dependent net acid load to the metabolic system. J Nutr 137:1491–1492. https://doi.org/10.1093/jn/137.6.1491
Remer T, Manz F, Alexy U et al (2011) Long-term high urinary potential renal acid load and low nitrogen excretion predict reduced diaphyseal bone mass and bone size in children. J Clin Endocrinol Metab 96:2861–2868. https://doi.org/10.1210/jc.2011-1005
Esche J, Shi L, Sánchez-Guijo A et al (2016) Higher diet-dependent renal acid load associates with higher glucocorticoid secretion and potentially bioactive free glucocorticoids in healthy children. Kidney Int 90:325–333. https://doi.org/10.1016/j.kint.2016.02.033
Krupp D, Hua Y, Esche J et al (2020) Renal biomarkers of acid excretion capacity: relationships with body fatness and blood pressure. Eur J Clin Nutr 74:76–82. https://doi.org/10.1038/s41430-020-0696-2
Remer T, Manz F (1995) Dietary protein as a modulator of the renal net acid excretion capacity: Evidence that an increased protein intake improves the capability of the kidney to excrete ammonium. J Nutr Biochem 6:431–437. https://doi.org/10.1016/0955-2863(95)00064-7
Manz F, Remer T, Decher-Spliethoff E et al (1995) Effects of a high protein intake on renal acid excretion in bodybuilders. Z Ernahrungswiss 34:10–15. https://doi.org/10.1007/BF01612779
Hua Y, Krupp D, Esche J et al (2019) Increased body fatness adversely relates to 24-hour urine pH during childhood and adolescence: evidence of an adipo-renal axis. Am J Clin Nutr 109:1279–1287. https://doi.org/10.1093/ajcn/nqy379
Berkemeyer S, Vormann J, Günther ALB et al (2008) Renal net acid excretion capacity is comparable in prepubescence, adolescence, and young adulthood but falls with aging. J Am Geriatr Soc 56:1442–1448. https://doi.org/10.1111/j.1532-5415.2008.01799.x
Maalouf NM, Cameron MA, Moe OW et al (2007) Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol 2:883–888. https://doi.org/10.2215/CJN.00670207
Hua Y, Herder C, Kalhoff H et al (2020) Inflammatory mediators in the adipo-renal axis: leptin, adiponectin, and soluble ICAM-1. Am J Physiol Renal Physiol 319:F469–F475. https://doi.org/10.1152/ajprenal.00257.2020
Maalouf NM, Cameron MA, Moe OW et al (2010) Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol 5:1277–1281. https://doi.org/10.2215/CJN.08331109
Hua Y, Esche J, Hartmann MF et al (2020) Cortisol and 11 beta-hydroxysteroid dehydrogenase type 2 as potential determinants of renal citrate excretion in healthy children. Endocrine 67:442–448. https://doi.org/10.1007/s12020-019-02151-0
Higgins C (2016) Urea and the clinical value of measuring blood ANS approved. https://acutecaretesting.org/-/media/acutecaretesting/files/pdf/urea-and-the-clinical-value-of-measuring-blood-ans-approved.pdf. Accessed 19 Jul 2022
Esche J, Krupp D, Mensink GBM et al (2018) Dietary potential renal acid load Is positively associated with serum uric acid and odds of hyperuricemia in the German adult population. J Nutr 148:49–55. https://doi.org/10.1093/jn/nxx003
Weinman EJ, Eknoyan G, Suki WN (1975) The influence of the extracellular fluid volume on the tubular reabsorption of uric acid. J Clin Invest 55:283–291. https://doi.org/10.1172/JCI107931
Golik A, Weissgarten J, Cotariu D et al (1993) Renal uric acid handling in non-insulin-dependent diabetic patients with elevated glomerular filtration rates. Clin Sci 85:713–716. https://doi.org/10.1042/cs0850713
Biradar MI, Chiang K-M, Yang H-C et al (2020) The causal role of elevated uric acid and waist circumference on the risk of metabolic syndrome components. Int J Obes 44:865–874. https://doi.org/10.1038/s41366-019-0487-9
Suijk DL, Smits MM, Muskiet MH et al (2020) Plasma uric acid and renal haemodynamics in type 2 diabetes patients. Nephrology (Carlton) 25:290–297. https://doi.org/10.1111/nep.13645
Schwingshackl L, Schünemann HJ, Meerpohl JJ (2020) Improving the trustworthiness of findings from nutrition evidence syntheses: assessing risk of bias and rating the certainty of evidence. Eur J Nutr. https://doi.org/10.1007/s00394-020-02464-1
Schünemann HJ, Cuello C, Akl EA et al (2019) GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol 111:105–114. https://doi.org/10.1016/j.jclinepi.2018.01.012
Acknowledgements
We would like to thank all panel members of the guideline on protein intake for their contributions to the methodological approach and specifically to the present manuscript. The following scientists deserve thanks for providing helpful remarks during guideline panel meetings and previous versions of this manuscript: Jürgen M. Bauer, Heiner Boeing, Anette E. Buyken, Julia Haardt, Sandrine Louis, Matthias B. Schulze, Gabriele I. Stangl, and Dorothee Volkert.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was partly funded by the German Federal Ministry of Food and Agriculture. The funder had no role in the decisions regarding data collection, analyses, interpretation of data, in the writing of the report or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Consortia
Contributions
NK, AMA, AL, and AS conducted the literature search, study selection, data extraction, and assessment of methodological quality and outcome-specific certainty of evidence. TR and RS evaluated the evidence and graded the overall certainty of evidence, which was finalised after discussion with all guideline panel members, and conducted critical appraisal of the kidney-related outcomes as appropriate renal function parameters. TR, RS, NK, and AS prepared the manuscript. All authors read, provided critical feedback, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
A list of possible conflicts of interest is provided in Supplementary Material S9.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Remer, T., Kalotai, N., Amini, A.M. et al. Protein intake and risk of urolithiasis and kidney diseases: an umbrella review of systematic reviews for the evidence-based guideline of the German Nutrition Society. Eur J Nutr 62, 1957–1975 (2023). https://doi.org/10.1007/s00394-023-03143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03143-7