Abstract
Purpose
Previous reports showed that some probiotics provide beneficial effects on various diseases including metabolic disorders. This study aimed to investigate the anti-obesity effects of Lactiplantibacillus (L.) plantarum SKO-001 (SKO-001), a probiotic strain newly isolated from Angelica gigas.
Methods
C57BL/6J mice were fed with high-fat diet (HFD, 60% fat) for four weeks, and then different doses of SKO-001 (n = 10 each group) were orally given for 12 weeks. Following treatment, body weight, fat weight, serum parameters and adipose and liver tissues were analyzed.
Results
SKO-001 (2 × 1010 CFU/day, per os) reduced body weight gain after 10th week of administration, accompanied by a reduction in body fat mass of mice. In the SKO-001-fed group, increased serum adiponectin, decreased leptin, insulin, total cholesterol, low-density lipoprotein cholesterol, free fatty acids, and triglyceride levels were observed. Hematoxylin and eosin staining of various fat depots showed that increased adipocyte size caused by HFD intake was markedly reduced and correlated with reduced mRNA levels of lipogenesis genes, including sterol regulatory element-binding protein-1c, peroxisome proliferator-activated receptor gamma, and CCAAT/enhancer binding protein alpha, and increased uncoupling protein 1 levels. Similarly, SKO-001 reduced lipid accumulation, decreased the mRNA levels of lipogenic genes, and reduced α-smooth muscle actin and collagen type 1 alpha 1 levels in the liver.
Conclusions
SKO-001 ameliorates obesity and related metabolic abnormalities in adipose and liver tissues, possibly via the regulation of lipid metabolism. Based on the results of the present study, SKO-001 may be applicable as an anti-obesity therapeutic or functional food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is the most important risk factor for metabolic disorders, including insulin resistance, type 2 diabetes mellitus, dyslipidemia, non-alcoholic fatty liver disease, and cardiovascular disease [1, 2]. Many factors, including unhealthy diet, genetic background, and sedentary lifestyle, lead to the development of obesity. Recently, alterations in the intestinal microbiota resulting from unhealthy diet have been shown to contribute to the pathogenesis of obesity [3]. Apart from bariatric surgery [4], several pharmacological agents are available for the treatment of obesity, among which appetite suppressants are popularly prescribed despite their central nervous system-related side effects [5].
The most prominent characteristic of obesity is excessive lipid accumulation in the body, resulting from an imbalance between lipogenesis and lipolysis under certain conditions [6]. The major tissues responsible for obesity are the adipose tissues, which are not mere depots for fat storage, but also act as an active endocrine organ and secrete various adipokines, including adiponectin, leptin, and resistin [7, 8]. Upon obesity development, adipocytes become enlarged, consequently provoking impaired insulin sensitivity and proinflammatory and diabetogenic conditions [9]. Interestingly, browning, the conversion of white adipocytes (enlarged spherical cells storing lipids) to brown-like adipocytes (small cells dissipating heat), reduces insulin resistance and obesity, and induction of uncoupling protein-1 (UCP-1) is a distinct marker of browning [10,11,12].
Chronic obesity results in a failure to manage the increased energy influx, leading to the storage of ectopic fat in tissues, including the liver and muscles. Abnormal hepatic lipid accumulation promotes non-alcoholic fatty liver disease, leading to liver fibrosis and cirrhosis [13]. The pathogenesis of hepatic fibrosis appears to be complex, where lipotoxicity caused by toxic lipid metabolites drives the progression of hepatic fibrosis via activation of hepatic stellate cells [14]. During liver fibrosis, activated hepatic stellate cells increase the levels of α-smooth muscle actin (α-SMA), remodeling the extracellular matrix by secreting matrix metalloproteases and depositing components, including collagen type 1 alpha 1 (Col1α1) [15]. Several mediators are involved in lipid metabolism, including sterol regulatory element-binding protein-1c (SREBP-1c) and CCAAT/enhancer binding protein alpha (C/EBPα), which are important transcription factors involved in the regulation of lipogenic gene mRNA expression. In addition, peroxisome proliferator-activated receptor gamma (PPARγ) and C/EBPα are key regulators of adipogenesis [16, 17].
Much effort has been focused on discovering natural products as supplementary or alternative anti-obesity agents, since these materials have long been used for treating various diseases without noticeable toxicity. Some probiotics confer healthy conditions to the host [18] by normalizing the impaired lipid and glucose metabolism, altering the composition of gut microbiota, and suppressing metabolic inflammation [19, 20]. For example, Lactiplantibacillus plantarum K21 for eight weeks of administration significantly reduced body weight gain, epididymal fat mass accumulation, and serum leptin levels in a high-fat diet (HFD)-induced obese mouse model [21]. Similarly, eight weeks of administration of L. plantarum LMT1-48 to HFD obese mice reduced body weight and abdominal fat volume concurrently with downregulation of lipogenic genes [22]. Reduction in body weight and body mass index by L. plantarum Dad-13 intake for 12 weeks has been reported [23]. Although the exact mechanisms and effects of probiotics are strain- and dose-dependent, several studies have demonstrated that probiotic intervention can be a safe treatment strategy for mitigating obesity [24,25,26]. Thus, in the present study, we investigated the anti-obesity effects of newly isolated L. plantarum SKO-001 (SKO-001) in HFD-induced obese mice.
Materials and methods
Reagents
Oligonucleotide primers specific for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), SREBP-1c, C/EBPα, PPARγ, UCP-1, αSMA, and Col1α1 were purchased from Bioneer (Daejeon, Korea). M-MLV reverse transcriptase and random oligonucleotide primers were from Promega (Fitchburg, WI, USA). TOP script RT Dry MIX was from Enzynomics Co., Ltd. (Daejeon, Korea). Thunderbird SYBR quantitative polymerase chain reaction (qPCR) Mix was from TOYOBO Co., Ltd. (Osaka, Japan). All other chemicals were from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Preparation of SKO-001
SKO-001 (Accession No. KCTC 14816BP) was isolated from Angelica gigas Nakai and prepared by Kolmar BNH Co., Ltd. (Seoul, Korea). Briefly, SKO-001 was anaerobically incubated in the culture medium (glucose, yeast extract, peptone, polysorbate 80, calcium chloride, magnesium sulfate, manganese (II) sulfate monohydrate, and LS-300) at 37 ± 2 ℃ for 16 h. After incubation, the cells were harvested via centrifugation at 9000×g, lyophilized, and stored at 4 ℃ until use. Freeze-dried SKO-001 powder was freshly suspended in autoclaved tap water daily for administration to animals.
In vivo studies
Six-week-old male C57BL/6J mice were purchased from Orient Bio (Seoul, Korea). The animals were acclimatized under the following conditions for seven days: temperature, 23 ± 2 ℃; humidity, 40–60%; circadian cycle, 12-h light/dark cycle. The mice were divided into the following five groups (n = 10/group): (1) control group, fed a normal chow diet (ND) and treated with vehicle (autoclaved tap water); (2) vehicle group, fed HFD (60% fat) for four weeks, followed by treatment with vehicle; (3) SKO-001-L group, fed HFD, followed by treatment with SKO-001 (5 × 109 CFU/day); (4) SKO-001-M group, fed HFD, followed by treatment with SKO-001 (1 × 1010 CFU/day); and (5) SKO-001-H group, fed HFD, followed by treatment with SKO-001 (2 × 1010 CFU/day). During the treatment period, body weight, food intake, and blood glucose levels were measured weekly at the same time of the day (between 10:00 and 11:00 AM). The amount difference between the supplied food and remaining food was considered as food intake. Blood was obtained by tail vein puncture, and analyzed glucose levels using Allmedicus GlucoDr Plus (Seoul, Korea). After 12 weeks of treatment, the animals were subjected to the assessment of fat and lean body mass using the 1H minispec system (Bruker BioSpin; Billerica, MA, USA). After overnight fasting, the animals were euthanized with isoflurane, and blood samples were collected via cardiac puncture and centrifuged at 3000×g for 15 min. Adipose and liver tissues from different depots were carefully excised and weighed. Part of the tissues were frozen in liquid nitrogen and stored at – 80 °C until further analysis. The other part of the adipose tissue was collected, fixed with 4% paraformaldehyde, and sectioned for UCP-1 immunostaining and hematoxylin and eosin (H&E) staining. Referring to the location of fat isolated from the mice, following regions were isolated; subcutaneous white adipose tissue (SAT) from the inguinal region; epididymal white adipose tissue (EAT) from the region located in the lower part of the abdomen and connected to the epididymis; visceral white adipose tissue (VAT) from the areas surrounding the intestine. All animal procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 2011) and were approved by the Animal Care and Use Committee of Gachon University, South Korea (approval No. LCDI-2021–0059).
RNA preparation and reverse-transcription-qPCR (RT-qPCR)
The isolated RNA from adipose and liver tissues was reverse-transcribed into complementary DNA using the TOP script RT Dry MIX and Oligo dT primers (Promega). RT-qPCR analysis was performed in triplicates for each sample. The expression of target genes was normalized to that of GAPDH. PCR conditions were as follows: 95 ℃ for 10 min (initial denaturation), followed by 45 cycles of 95 ℃ for 15 s (denaturation), 53 ℃ for 1 min (annealing), and 72 ℃ for 30 s (elongation). The sequences of the primer sets used in this study are listed in Table 1.
H&E staining and UCP-1 immunostaining of adipose tissues
Paraffin-embedded tissue Sects. (4–6 μm) were deparaffinized, rehydrated, permeabilized with 0.1% Triton X-100 (1 h, room temperature), and washed with phosphate-buffered saline (PBS). The tissue sections were stained with H&E, and images were captured using an Axio Imager Z1 microscope (Carl Zeiss Microscopy, Oberkochen, Germany). For UCP-1 immunostaining, the sections were incubated with primary polyclonal antibodies against UCP-1 (Abcam, Cambridge, UK; diluted 1:200) at 4 °C overnight. After washing with PBS, the sections were incubated with Alexa Fluor 488 (Invitrogen, Waltham, MA, USA; diluted 1:300). After washing with wash buffer, the sections were mounted with VECTASHIELD mounting medium containing 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI; Vector Laboratories, Newark, CA, USA) and examined under a Zeiss LSM700 confocal microscope (Carl Zeiss Microscopy). Using the Viewpoint-Viewer software (PreciPoint, Freising, Germany), the size of adipocytes in the field was determined.
α-SMA immunostaining of liver tissues
Liver tissues fixed in 10% neutral buffered formalin were embedded in paraffin after washing with PBS. The Sects. (4–6 μm) were incubated with primary polyclonal antibodies against α-SMA (Novus Biologicals, Littleton, CO, USA; diluted 1:200) at 4 °C overnight. After washing with PBS, sections were incubated with Alexa Fluor 488 (Invitrogen; diluted 1:300). After washing with wash buffer, the sections were mounted with VECTASHIELD mounting medium containing DAPI and examined using a Zeiss LSM700 confocal microscope.
Oil Red O staining
Liver tissues were fixed in 10% neutral buffered formalin for frozen sectioning (– 15 °C). Frozen Sects. (20-µm thickness) were incubated with 60% isopropanol for 5 min and stained with 0.3% Oil Red O/60% isopropanol solution for 10 min after complete drying, followed by five dips of alum hematoxylin. After rinsing with distilled water, images of the lipid droplets were visualized and captured under an Axio Imager Z1 microscope.
Analysis of serum biochemical markers
Serum levels of various markers were determined using commercial kits according to the protocols provided by each vendor: free fatty acid (FFA; Abcam), low-density lipoprotein cholesterol (LDL-C)/high-density lipoprotein cholesterol (HDL-C; Abcam), insulin (ALPCO, Macedon, NY, USA), adiponectin (ALPCO), triglyceride (Abbkine Scientific Co. Ltd., CA, USA), total cholesterol (TC; Abbkine), FFA (Abbkine), and leptin (Abbkine).
Statistical analysis
The results are expressed as the mean ± standard deviation for each group. The means between the treatment groups were compared using Student’s t-test or two-way analysis of variance. Statistical significance between groups was considered at p < 0.05.
Results
SKO-001 suppresses HFD-induced increase in body and fat weights
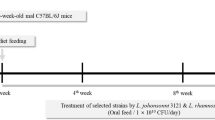
To determine the anti-obesity effects of SKO-001, C57BL/6J mice were fed a HFD (60% fat) for four weeks, followed by oral administration of different doses of SKO-001 (SKO-001-L, 5 × 109 CFU/day; SKO-001-M, 1 × 1010 CFU/day; SKO-001-H, 2 × 1010 CFU/day) once daily for 12 weeks (Fig. 1A). As shown in Fig. 1B, HFD feeding induced significant body weight gain throughout the experimental period compared with ND feeding. Upon oral administration of SKO-001, body weight gain was reduced starting from 10th week administration (SKO-001-H). At the end of the experiments, the vehicle group (HFD group) gained 43.1 ± 3.76 g, while SKO-001-H-fed group gained 37.7 ± 3.45 g body weight (12.7% decrease vs. HFD group; p < 0.05). Meanwhile, little alteration in feed intake and non-fasting glucose levels were observed in the SKO-001-treated groups, although feed intake between the ND- and HFD-fed groups was considerably different (Fig. 1C, D). Consistent with the effects of SKO-001 on body weight, the HFD-induced increase in body fat mass measured by minispec was also significantly suppressed in the SKO-001-H group without any change in whole-body lean mass (Fig. 2), suggesting that decreased body weight gain by SKO-001 may be attributed to the selective reduction of fat mass.
Effects of SKO-001 on body weight, food intake, and plasma glucose levels in HFD-induced obese mice. C57BL/6J mice (seven weeks old, male) were fed either a normal chow diet (ND) or HFD for four weeks. HFD-induced obese mice were orally administered SKO-001 (three different doses; once daily) for 12 weeks. Experimental scheme is shown (A). Body weight (B), food intake (C), and plasma glucose levels (D) were measured at the indicated days (n = 10/group). Values are represented as the mean ± standard deviation (SD). ns not significant. ***p < 0.001 vs. ND condition. #p < 0.05 vs. HFD condition
Effect of SKO-001 on fat mass in HFD-induced obese mice. After 12 weeks treatment of mice with SKO-001, the animals were subjected to fat mass measurements. Fat and lean body mass were assessed using the 1H minispec system. Values are represented as the mean ± SD. ***p < 0.001 vs. ND condition. #p < 0.05 vs. HFD condition
SKO-001 improves blood parameters in HFD-induced obese mice
Reduced body and fat weights are associated with increased whole-body insulin sensitivity. Thus, the plasma levels of various cytokines were determined after 12 weeks of oral administration of SKO-001 to examine its effects on insulin sensitivity. Two important cytokines related to insulin sensitivity, namely, adiponectin and leptin, were altered by HFD feeding: increased leptin and reduced adiponectin levels (Fig. 3A). The reduced levels of adiponectin induced by HFD feeding were recovered in a dose-dependent manner by SKO-001. Conversely, the HFD-induced leptin levels were normalized to levels similar to those in the ND group after SKO-001 treatment (Fig. 3A). While SKO-001 had little effect on plasma glucose levels compared with the HFD group, plasma insulin levels were markedly reduced by SKO-001 treatment (Fig. 3A), suggesting that SKO-001 may improve insulin resistance developed by chronic HFD feeding. Moreover, overall lipid profiles were ameliorated in SKO-001-treated groups, that is, the levels of TC, LDL-C, FFA, and triglycerides decreased markedly in SKO-001-treated groups than in the HFD group (Fig. 3B), suggesting that SKO-001 is effective for recovering abnormal lipid metabolism induced by HFD feeding.
Effects of SKO-001 on serum biochemical parameters in HFD-induced obese mice. HFD-induced obese mice were orally administered SKO-001 for 12 weeks. After fat mass measurement, the mice were subjected to overnight fasting, and blood samples from each mouse were taken via cardiac puncture the next morning. Various serum parameters were determined using enzyme-linked immunosorbent assay (ELISA) kits as indicated in the Materials and methods section. A Adiponectin, leptin, and insulin levels. B Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), free fatty acid (FFA), triglyceride, and glucose levels. Values are represented as the mean ± SD. ***p < 0.001 vs. ND condition. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. HFD condition
SKO-001 ameliorates lipid metabolism-related parameters in adipose tissues
Analysis of metric and biochemical parameters revealed that SKO-001 improved HFD-induced obesity. To elucidate the mechanisms involved in SKO-001 action, the effects of SKO-001 on adipose tissue were analyzed after 12 weeks of administration in HFD-induced obese mice. First, H&E staining of various fat depots revealed that SKO-001 supplementation reduced the average adipocyte size in all adipose tissues (SAT, VAT, and EAT) compared with those in the HFD group, suggesting that white adipocytes containing large lipid droplets were more likely to beige adipocytes upon SKO-001 administration (Fig. 4A, B). Correspondingly, the mRNA levels of UCP-1, a representative marker of beige adipocytes, were elevated by SKO-001 in all adipose tissues (SAT, VAT, and EAT) (Fig. 5A), as revealed by UCP-1 immunostaining (Fig. 5B). Second, changes in the mRNA expression of lipogenesis-related genes in adipose tissues were analyzed using RT-qPCR. As shown in Fig. 6A, the mRNA expression of adipogenic transcription factors, including PPARγ and C/EBPα, were significantly increased in the HFD group than in the ND group. SKO-001 treatment reduced PPARγ and C/EBPα levels in SAT. Furthermore, significantly lower mRNA levels of SREBP-1c were noticed in the SKO-001-treated groups. SKO-001 exhibited similar effects in VAT and EAT (Fig. 6B, C). Correspondingly, all protein levels were also reduced (Fig. 6A–C right panels), implying that SKO-001 had suppressive effects on HFD-induced lipogenesis in WATs, regardless of the source of adipose tissues.
Effects of SKO-001 on adipocyte size in the adipose tissues of HFD-induced obese mice. After cardiac puncture to obtain blood samples from each group, the adipose tissues were isolated, and paraffin-embedded tissue sections were stained with hematoxylin and eosin (H&E). Images were captured under the microscope, Axio imager Z1 (A) (Scale bar = 50 μm, 200 × magnification). The cell size in each group was estimated using Viewpoint-viewer software program (B). SAT; subcutaneous white adipose tissue, VAT; visceral white adipose tissues, EAT; epididymal white adipose tissue. Values are represented as the mean ± SD. ***p < 0.001 vs. ND condition. #p < 0.05, ###p < 0.001 vs. HFD condition
Effects of SKO-001 on UCP-1 expression in the adipose tissues of HFD-induced obese mice. Adipose tissues isolated from each group were subjected to RT-qPCR analysis. The mRNA levels of UCP-1 were determined using real-time RT-qPCR (repeated three times, each in triplicate; A. UCP-1 immunostaining was carried out using paraffin-embedded tissue sections (B; Scale bar = 50 μm, 200 × magnification). Values are represented as the mean ± SD. *p < 0.05, **p < 0.01 vs. ND condition. #p < 0.05, ##p < 0.01 vs. HFD condition
Effects of SKO-001 on the mRNA expression of genes involved in lipogenesis in the adipose tissues of HFD-induced obese mice. Adipose tissues isolated from each group were subjected to RT-qPCR analysis. The mRNA levels of lipogenesis markers (SREBP-1c, PPARγ, and C/EBPα) were determined via real-time RT-qPCR (repeated three times, each in triplicate; A–C left panel). Protein levels were determined via western blotting analysis (A–C right panel). Values are represented as the mean ± SD. *p < 0.05, **p < 0.01 vs. ND condition. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. HFD condition
SKO-001 improves HFD-induced liver impairments
Chronic obesity leads to accumulation of ectopic fat in tissues, notably in the liver. To further investigate the anti-lipogenic effects of SKO-001, hepatic lipid levels were measured using Oil Red O staining, and the results indicated that SKO-001 also reduced lipid accumulation in the liver (Fig. 7A). Likewise, the mRNA and protein levels of lipogenesis markers were also lower in the SKO-001-treated groups than in the HFD group, suggesting that SKO-001 improved the lipid metabolism profile in the liver, consistent with its effects on plasma determinants (Fig. 7B, C). Moreover, the mRNA levels of fibrosis markers, including αSMA and Col1α1, were decreased by SKO-001 (Fig. 7E), as revealed via immunostaining (Fig. 7D), which implies that SKO-001 may also have beneficial effects on liver fibrosis and cirrhosis.
Effects of SKO-001 on liver tissues in HFD-induced obese mice. Liver tissues isolated from each group were subjected to Oil Red O staining (A) and α-SMA immunostaining (D) (Scale bar = 50 μm, 200 × magnification). The mRNA (B) and protein (C) levels of lipogenesis and fibrosis markers (E) were determined using real-time RT-qPCR (repeated three times, each in triplicate). Values are expressed as the mean ± SD. ***p < 0.001 vs. ND condition. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. HFD condition
Discussion
Despite the drastic increase in the prevalence of obesity worldwide, effective therapeutic strategies to combat it are still lacking. Currently, several appetite suppressants are prescribed to treat severe obesity and related metabolic disorders [5]. Meanwhile, efforts are being made to overcome the adverse effects of existing drugs and expand the research on the discovery of novel therapeutic targets from natural sources. Some probiotics exert beneficial effects in reducing the body weight in rodents, and may possibly be used to treat human obesity [18, 27]. Lactobacilli and Bifidobacteria are the most well-studied probiotic strains that are effective against obesity and associated diseases [6].
In the present study, we discovered SKO-001, a probiotic strain isolated from A. gigas, as a possible anti-obesity agent and investigated its effects in HFD-induced obese mice. Based on the preliminary toxicity studies showing that any toxicological signs were not observed up to 6 × 1010 CFU/day (results not shown), three different doses of SKO-001 were selected and orally administered to mice along with the HFD for 12 weeks. The suppressive effects of SKO-001 on whole body weight appeared to be mild, and visible only at SKO-001-H (2 × 1010 CFU/day, at high dose), while its effects were limited to fat mass without any noticeable change in lean mass, suggesting that SKO-001 selectively works on fat mass reduction. In contrast, other obesity-induced parameters were significantly reversed by all doses of SKO-001, including SKO-001-L (low-dose). HFD-induced insulin resistance appears to be ameliorated by SKO-001, as evidenced by the increased plasma adiponectin levels and decreased leptin and insulin levels, in line with previous reports showing that Lactobacillus gasseri BNR17 induces weight reduction accompanied by the normalization of plasma levels of various cytokines [27].
Adipose tissue functions as an energy reservoir. However, obese adipose tissue fails to handle with excess fat, becoming dysfunctional. Notably, obesity-induced adipocyte hypertrophy triggers low-grade inflammation and subsequent insulin resistance [28]. Adipose browning, which refers to either the de novo differentiation of precursor cells to brown-like adipocytes or the trans-differentiation of white adipocytes to brown-like adipocytes, may offer a novel means of treating obesity and related metabolic diseases [10,11,12]. In the present study, SKO-001 administration resulted in reduced adipocyte size, along with increased levels of UCP-1, an important marker of browning, suggesting that SKO-001 may induce adipocyte browning, which may partly contribute to its anti-obesity effects by stimulating energy metabolism.
To further delineate the mechanism of action of SKO-001, we analyzed the expression of genes associated with lipogenesis in adipose tissue. The levels of SREBP-1c, which increases glycolysis and lipogenesis [17], were largely decreased by SKO-001 in a dose-dependent manner. PPARγ is a major transcription factor that modulates both glucose and lipid metabolism, and its expression in adipose tissue is associated with HFD-induced adipocyte hypertrophy and insulin resistance [29, 30]. SKO-001 treatment reverses the HFD-induced increase in PPARγ expression. Consistent with our findings, reduced SREBP-1c and PPARγ2 expression were observed upon weight loss in women with obesity [31] and in HFD-induced obese mice [32]. In addition, mRNA expression of C/EBPα, a key regulator of adipogenesis and lipid accumulation [16], were completely suppressed by SKO-001, suggesting that inhibition of SREBP-1c and C/EBPα mRNA expression by SKO-001 resulted in reduced lipid accumulation. Interestingly, C/EBPα binds to the promoter and modulates the expression of leptin, which plays an important role in body weight homeostasis [33]. Consistently, reduced leptin levels were observed in the SKO-001-treated groups in our study, which may be linked to the anti-obesity effects of SKO-001. Similar effects of SKO-001 were observed in in vitro study that SKO-001 suppressed differentiation of 3T3-L1 preadipocytes, in parallel with reduced expression of lipogenesis genes (Suppl. Figure 5).
Increased fat accumulation in obesity is connected with dyslipidemia, collectively implying increased triglyceride, LDL-C, and TC levels, and decreased HDL-C levels and subsequently increasing the risk of coronary artery disease [4]. The results of the present study showed that SKO-001 improved HFD-induced changes in serum lipids by decreasing triglyceride, LDL-C, and TC levels, while concurrently increasing HDL-C levels. Furthermore, chronic obesity leads to the accumulation of ectopic fat in tissues, including the liver and muscles, resulting from the failure of the adipose tissue to handle the increased energy influx [13]. Indeed, SKO-001 significantly restored HFD-induced ectopic lipid accumulation in the liver, along with reduced mRNA expression of SREBP-1c, C/EBPα, and PPARγ. Additionally, SKO-001 reduced the mRNA levels of two important fibrosis markers, α-SMA and Col1α1, along with αSMA protein levels, as determined by α-SMA immunostaining, suggesting that SKO-001 may also be applicable to hepatic fibrosis and cirrhosis.
Long term diet containing high fat causes microbial dysbiosis, which can be ameliorated by probiotics including L. plantarum. For example, L. plantarum 1201 rebalanced the ratio of firmicutes/bacteroidetes, alleviating high-salt induced colitis [35]. Similarly, L. plantarum A29 increased percentage of firmicutes phylum compared with high-fat diet alone, which lowered body weights of HFD-induced obese mice [36]. Future studies to investigate the effects of SKO-001 on gut microbiota composition would be required. On the other hand, several metabolites produced by probiotics, such as lactic acid and short-chain fatty acids, also have shown beneficial effects against obesity and its related disorders [37]. It may be possible SKO-001 metabolites also contribute to the anti-obesity effects of SKO-001, which should be identified in future studies.
In summary, we demonstrated that SKO-001 suppresses HFD-induced body weight gain, improves serum biochemical parameters, and reduces ectopic lipid accumulation in the liver. The anti-obesity effects of SKO-001 may be due to the transcriptional downregulation of lipogenesis-related gene expression and induction of adipocyte browning. In addition, SKO-001 exerts beneficial effects on hepatic fibrosis. However, the detailed mechanisms of action of SKO-001 in adipose and liver tissues remain to be elucidated. It should also be clarified whether the anti-fibrotic effects of SKO-001 are independent of its effects on adipose tissue. Collectively, SKO-001 may be used as a novel therapeutic and/or dietary agent for ameliorating human obesity and its related metabolic disorders. Although the adverse effects of probiotics are rarely reported, the safety profile and human efficacy of SKO-001 should be investigated in future studies.
Data availability
All data generated in this study are contained within the article or supplementary material file.
References
Keller KB, Lemberg L (2003) Obesity and the metabolic syndrome. Am J Crit Care 12:167–170 (PMID: 12615276)
Grundy SM (2004) Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89:2595–2600. https://doi.org/10.1210/jc.2004-0372
Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101:15718–15723. https://doi.org/10.1073/pnas.0407076101
Dixon JB, le Roux CW, Rubino F, Zimmet P (2012) Bariatric surgery for type 2 diabetes. Lancet 379:2300–2311. https://doi.org/10.1016/S0140-6736(12)60401-2
Srivastava G, Apovian C (2018) Future pharmacotherapy for obesity: new anti-obesity drugs on the horizon. Curr Obes Rep 7:147–161. https://doi.org/10.1007/s13679-018-0300-4
Tang C, Kong L, Shan M, Lu Z, Lu Y (2021) Protective and ameliorating effects of probiotics against diet-induced obesity: A review. Food Res Int 147: 110490. https://doi.org/10.1016/j.foodres.2021.110490
Coelho M, Oliveira T, Fernandes R (2013) Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 9:191–200. https://doi.org/10.5114/aoms.2013.33181
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clinical Endo Metabolism 89:2548–2556. https://doi.org/10.1210/jc.2004-0395
Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, Beguinot F, Miele C (2019) Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci 20:2358. https://doi.org/10.3390/ijms20092358
Poher AL, Veyrat-Durebex C, Altirriba J, Montetn X, Colin DJ, Caillon A, Lyautey J, Jeanrenaud FR (2015) Ectopic UCP1 overexpression in white adipose tissue improves insulin sensitivity in Lou/C rats, a model of obesity resistance. Diabetes 64:3700–3712. https://doi.org/10.2337/db15-0210
Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G (2014) A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab 20: 433–447. https://doi.org/10.1016/j.cmet.2014.06.011
Kim SH, Plutzky J (2016) Brown fat and browning for the treatment of obesity and related metabolic disorders. Diabetes Metab J 40:12–21. https://doi.org/10.4093/dmj.2016.40.1.12
Lomonaco R, Sunny NE, Bril F, Cusi K (2013) Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs 73: 1–14. https://doi.org/10.1007/s40265-012-0004-0
Marra F, Svegliati-Baroni G (2018) Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol 68:280–295. https://doi.org/10.1016/j.hep.2017.11.014
Zhang CY, Yuan WG, He P, Lei JH, Wang CX (2016) Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol 22:10512–10522. https://doi.org/10.3748/wjg.v22.i48.10512
Pedersen TA, Bereshchenki O, Garcia-Silva S, Ermakova O, Kurz E, Mandrup S, Porce BT, Nerlov C (2007) Distinct C/EBPa motifs regulate lipogenic and gluconeogenic gene expression in vivo. EMBO J 26:1081–1093. https://doi.org/10.1038/sj.emboj.7601563
Ferre P, Foufelle F (2007) SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res 68:72–82. https://doi.org/10.1159/000100426
Tang C, Lu Z (2019) Health promoting activities of probiotics. J Food Biochem 43: e12944. https://doi.org/10.1111/jfbc.12944
Kobyliak N, Conte C, Cammarota G, Haley AP, Styriak I, Gaspar L, Fusek J, Rodrigo L, Kruzliak P (2016) Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab 13:14. https://doi.org/10.1186/s12986-016-0067-0
Li Y, Liu T, Zhang X, Zhao M, Zhang H, Feng F (2019) Lactobacillus plantarum helps to suppress body weight gain, improve serum lipid profile and ameliorate low-grade inflammation in mice administered with glycerol monolaurate. J Func Foods 53:54–61. https://doi.org/10.1016/j.jff.2019.12.015
Wu CC, Weng WL, Lai WL, Tsai HP, Liu WH, Lee MH, Tsai YC (2015) Effect of Lactobacillus Plantarum strain K21 on high fat diet-fed obese mice. Evid Based Complement Alternat Med 391767. https://doi.org/10.1155/2015/391767
Choi WJ, Dong HJ, Jeong HU, Ryu DW, Song SM, Kim YR, Jung HH, Kim TH, Kim YH (2020) Lactobacillus plantarum LMT1–48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Sci Rep 10: 869. https://doi.org/10.1038/s41598-020-57615-5
Rahayu ES, Mariyatun M, Manurung NEP, Hasan PN, Therdtatha P, Mishima R, Komalasari H, Mahfuzah NA, Pamungkaningtyas FH, Yoga WK (2021) Effect of probiotic Lactobacillus plantarum Dad-13 powder consumption on the gut microbiota and intestinal health of overweight adults. World J Gastroenterol 27: 107–128. https://doi.org/10.3748/wjg.v27.i1.107
Barathikannan K, Chelliah R, Rubab M, Daliri EBM, Elahi F, Kim DH, Agastian P, Oh SY, Oh DH (2019) Gut microbiome modulation based on probiotic application for anti-obesity: a review on efficacy and validation. Microorganisms 7: 456. https://doi.org/10.3390/microorganisms7100456
Ji Y, Chung YM, Park S, Jeong D, Kim B, Holzapfel WH (2019) Dose-dependent and strain-dependent anti-obesity effects of Lactobacillus sakei in a diet induced obese murine model. Peer J 7: e6651. https://doi.org/10.7717/peerj.6551
Karimi G, Sabran MR, Jamaluddin RJ (2015) The anti-obesity effects of Lactobacillus casei strain Shirota versus Orlistat on high fat diet-induced obese rats. Food Nutr Res 59:29273. https://doi.org/10.3402/fnr/.v59.29273
Kang JH, Yun SI, Park MH, Park JH, Jeong PA, Park HO (2013) Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. Plos One 8: e54617. https://doi.org/10.1371/journal.pone.0054617
Shoelson SE, Lee J, Yuan M (2003) Inflammation and the IKKb/IkB/NF-kB axis in obesity- and diet-induced IR. Int J Obes Relat Metab Disord 27: S49-S52. https://doi.org/10.1038/sj.ijo.0802501
Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T (1999) PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 4:597–609. https://doi.org/10.1016/s1097-2765(00)80210-5
Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA (2005) Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA 102:6207–6212. https://doi.org/10.1073/pnas.0306743102
Ribot J, Rantala M, Kesaniemi VA, Palou A, Savolainen MJ (2001) Weight loss reduces expression of SREBP1c/ADD1 and PPARgamma2 in adipose tissue of obese women. Pflugers Arch 441:498–505. https://doi.org/10.1007/s004240000454
Park YJ, Seo DW, Gil TY, Cominguez DC, Lee H, Lee DS, Han I, An HJ (2020) Pharmacological properties of a traditional Korean formula Bojungchiseup-tang on 3T3-L1 preadipocytes and hifh-fat diet-induced obesity mouse model. Biomed Res Int 2020:8851010. https://doi.org/10.1155/2020/8851010
Miller SG, Vos PD, Guerre-Millo M, Wong E, Herman T, Staels B, Briggs MR, Auwerx J (1996) The adipocyte specific transcription factor C/EBPa modulates human ob gene expression. Proc Natl Acad Sci USA 93:5507–5511. https://doi.org/10.1073/pnas.93.11.5507
Carr MC, Brunzell JD (2004) Abdominal obesity and dyslipidemia in the metabolic syndrome: Importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk. J Clin Endocrinol Metab 89:2601–2607. https://doi.org/10.1210/jc.2004-0432
Chen S, Ren Z, Huo Y, Yang W, Peng L, Lv H, Nie L, Wei H, Wan C (2022) Targeting the gut microbiota to investigate the mechanism of Lactiplantibacillus plantarum 1201 in negating colitis aggravated by a high-salt diet. Food Res Int 162 (PtA): 112010. https://doi.org/10.1016/j.foodres.2022.112010
Soundharrajan I, Kuppusamy P, Srisesharam S, Lee JC, Sivanesan R, Kim D, Choi KC (2020) Positive metabolic effects of selected probiotic bacteria on diet-induced obesity in mice are associated with improvement of dysbiotic gut microbiota. FASEB J 34:12289–12307. https://doi.org/10.1096/fj.202000971R
Tnag C, Meng F, Pang X, Chen M, Zhou L, Lu Z, Lu Y (2020) Protective effects of Lactobacillus acidophilus NX2–6 against oleic acid-induced steatosis, mitochondrial dysfunction, endoplasmic reticulum stress and inflammatory responses. J Funct Foods 74: 104206. https://doi.org/10.1016/j.jff2020.104206
Funding
This research was supported by a grant from Kolmar BNH Co., Ltd., Korea (Grant no. 202102730001).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, MJC, HY, JIK, HS, and JGK; data curation, MJC and HY; writing—review and editing, MJC, SKK, HSL and HGC; supervision, HGC. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
About this article
Cite this article
Choi, M.J., Yu, H., Kim, J.I. et al. Anti-obesity effects of Lactiplantibacillus plantarum SKO-001 in high-fat diet-induced obese mice. Eur J Nutr 62, 1611–1622 (2023). https://doi.org/10.1007/s00394-023-03096-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03096-x