Abstract
Purpose
To study the intake and sources of vitamin D and determinants of serum 25-hydroxyvitamin D (S-25(OH)D) in Finnish adolescents.
Methods
We studied 265 adolescents (117 girls) aged 15–17 years attending 8-year examinations of the PANIC Study, assessed diet using food records and other lifestyle factors by questionnaires, and analyzed S-25(OH)D by chemiluminescence immunoassay and determinants of S-25(OH)D using multivariate linear regression.
Results
Mean (standard deviation) of total vitamin D intake from food and supplements was 19.2 (13.1) µg/d, and that of dietary vitamin D intake was 9.9 (5.4) µg/d. Milk fortified with vitamin D was the main dietary source of vitamin D, providing 45% of daily intake. Altogether, 29% of the adolescents used no vitamin D supplements and 25% did not meet the recommended total vitamin D intake of 10 µg/d. Mean (standard deviation) of S-25(OH)D was 62.0 (18.8) nmol/l, and S-25(OH)D was < 50 nmol/l in 29.5% of the adolescents. Vitamin D intake from supplements was the main determinant of S-25(OH)D (β = 0.465, p < 0.001), followed by consumption of milk products (β = 0.251, p < 0.001), consumption of meat products (β = 0.179, p = 0.002), travels to sunny countries (β = 0.178, p = 0.002), and average daylight time (β = 0.162, p = 0.004).
Conclusion
Most of the adolescents had vitamin D intake at the recommended level, although a fourth did not meet the recommended total vitamin D intake of 10 µg/d and almost a third had S-25(OH)D < 50 nmol/l. More attention should be paid to the sufficient intake of vitamin D in adolescents who do not use vitamin D supplements or fortified milk products.
Trial registration
ClinicalTrials.gov: NCT01803776, registered March 3, 2013.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D is essential in childhood growth and bone mineralization [1]. Vitamin D may also have beneficial effects on other health outcomes than bone health, including respiratory infections, autoimmune diseases, cardiovascular risk factors, some types of cancer, and mortality, even though evidence on these health effects remains inconclusive [2, 3].
The recommendations for vitamin D intake and the definitions of vitamin D deficiency are based on a serum concentration of 25-hydroxyvitamin D (25(OH)D) [2]. The limit for vitamin D deficiency varies between 25 and 50 nmol/l, and the lower limit for sufficiency between 50 and 75 nmol/l [1, 4,5,6,7,8,9]. Such a variation in the recommendations for serum 25(OH)D concentration is partly related to different target groups. Moreover, some recommendations take only the effects of vitamin D on bone health into account, whereas others consider also other possible health effects. The recommendations for vitamin D intake targeted for general populations of different ages typically vary between 10 and 20 µg/d [6,7,8,9], and the recommendations aiming at higher serum levels above 75 nmol/l vary between 10 and 50 µg/d for different age groups [5]. The international comparison of serum 25(OH)D levels across studies has been challenging due to differences between serum 25(OH)D assays. The Vitamin D Standardization Program was therefore established in 2010 as an international collaborative effort to standardize the laboratory measurement of vitamin D status so that the results would be comparable and independent of the location and laboratory procedures used [10].
There are few natural sources of vitamin D, including fish, egg yolk, and some mushroom [7], and certain foods may therefore need to be fortified to increase vitamin D intake at the population level [7, 11]. Accordingly, the recommendation for vitamin D fortification of liquid milk products was increased from 0.5 to 1 µg/100 g and for spreads from 10 to 20 µg/100 g in Finland in 2010 [12]. Moreover, the Finnish recommendation for vitamin D supplement use was increased to 7.5 µg/d throughout the year for children and adolescents aged 2–17 years in 2011, and the recommendation for total vitamin D intake was increased to 10 µg/d for individuals aged 2–74 years in 2014 [13]. We found that more than half of the Finnish children 6–8 years of age had insufficient vitamin D intake and fifth of the children had a serum 25(OH)D concentration < 50 nmol/l at the baseline examinations of the Physical Activity and Nutrition in Children (PANIC) Study in 2007–2009 [14], which was prior to the increases in Finnish recommendations for vitamin D intake, supplementation use, and fortification. However, after these changes in the Finnish recommendations, the total intake of vitamin D and serum 25(OH)D concentrations have increased in adults [15,16,17], and total vitamin D intake has been sufficient in the majority of children [18, 19].
Adolescents appear to have a higher risk of low serum 25(OH)D compared with children and adults [20, 21], and it is important to understand why adolescents do not meet the vitamin D recommendations. However, there are no reports on vitamin D intake and serum 25(OH)D concentrations in adolescents after the increases of the recommended vitamin D intake, supplement use, and fortification in Finland. For these reasons, we studied total and dietary vitamin D intake, vitamin D supplementation use, the sources of vitamin D, serum 25(OH)D concentrations, and the cross-sectional determinants of serum 25(OH)D in a population-based sample of Finnish adolescents participating in the 8-year examinations of the PANIC Study in 2016–2018 using a standardized assay to measure serum 25(OH)D concentrations.
Methods
Study design and participants
The present analyses are based on the cross-sectional data from the 8-year examinations of the PANIC Study that is an 8-year controlled lifestyle intervention study aimed at investigating the effects of a combined diet and physical activity (PA) intervention on cardiometabolic risk factors in a population-based sample of children from the city of Kuopio, Finland. The details of the study protocol, including sample size calculations, participant recruitment, interventions, and assessments, have been described in detail previously [22, 23]. Briefly, we invited 736 children 6–9 years of age who started the first grade in 16 primary schools of Kuopio in 2007–2009 to the baseline examinations of the PANIC Study, and 504 children were included in the lifestyle intervention study. The participants did not differ in age, sex, or body mass index-standard deviation score (BMI-SDS) from all children who started first grade in the City of Kuopio in 2007–2009 based on data from the standard school health examinations performed for all Finnish children before the first grade. The lifestyle intervention was focused on improving diet quality, increasing PA, and decreasing sedentary time according to the Finnish dietary and PA recommendations [24, 25] and was not specifically targeted on increasing total vitamin D intake. Of the 504 children who participated in the baseline examinations, 277 (55%) attended the 8-year examinations between January 2016 and January 2018. Those who participated in the 8-year examinations did not differ in age, BMI-SDS, vitamin D intake from food or supplements, or the distribution of sex or study groups at baseline from those who dropped out (data not shown). Altogether 265 adolescents (117 girls, 148 boys) had data on serum 25(OH)D, had no diseases or medications known to affect serum 25(OH)D, and were thus included in these analyses based on the cross-sectional data from the 8-year examinations of the PANIC Study. Of these adolescents, 97.3% were Caucasian.
Assessment of food consumption and nutrient intake
We assessed the consumption of foods, energy intake, and the dietary intake of vitamin D using food records of 4 days in 213 (96%) adolescents or 3 days in 8 (4%) adolescents [23]. The clinical nutritionists who were trained based on the protocol of the study gave the instructions about filling out the food records to the participants at the research site during the study visits. The adolescents were instructed to record their food and drink consumption using household or other measures, such as tablespoons, deciliters, and centimeters. They were also provided a picture booklet of portion sizes to help estimate portion sizes. The clinical nutritionists checked the returned food records, together with the adolescents and completed any missing information. If any missing information remained, the researchers used standard portion sizes and quality details according to the study protocol. Food consumption and nutrient intakes were assessed using the Micro Nutrica® dietary analysis software, version 2.5 (The Social Insurance Institution of Finland, Turku, Finland). The software is based on national analyses and international food composition tables [26]. Moreover, a clinical nutritionist updated the software by adding new food products with their actual nutrient content based on the updated data in the Finnish food composition database [27] or received from the food producers.
Milk was generally fortified with vitamin D with mostly 1 μg/100 g as recommended by the National Nutrition Council [12], but some milk products contained even 2 μg of vitamin D/100 g. In Finland, vitamin D fortification of foods is voluntary, except for fat-free homogenized milk (including organic fat-free homogenized milk), for which fortification of at least 1 µg/d has been mandatory since 2016 [28]. Some of the yoghurts and other sour milk products were fortified with vitamin D mostly with 1 μg/100 g. Vegetable oil-based spreads were generally fortified with vitamin D with 20 μg/100 g according to the recommendations [12]. Other fat products included mainly vegetable oil-based baking products, many of which are fortified with vitamin D in Finland, and coconut oil that is not fortified with vitamin D. Fish products included fresh fish, shellfish and processed fish. Plant based milk-type products, such as oat and soya drink, are usually fortified with vitamin D. Vitamin supplements were not included in the food records.
Assessment of supplement use
The use of vitamin and mineral supplements was assessed by a questionnaire in which we asked if the adolescents had used any supplement containing vitamin D during the past year, the name of the supplement product used, the dose of vitamin D in the supplement in µg, how many times per week that supplements was used, in which month did the adolescent begin to use the supplement and in which month did the adolescent quit to use the supplement (if not used year-round). Vitamin D supplements and multivitamin supplements containing vitamin D were combined for the analyses. The average daily dose of vitamin D during a year was calculated by combining the information on the frequency of supplement use, the estimated number of weeks per year of supplement use, and the dosage by one researcher (SS).
Assessment of physical activity and sedentary time
PA and sedentary time were assessed by the PANIC Physical Activity and Hobby Questionnaire. The types of PA included commuting to and from school, physical education at school, PA during recess, organized sports, competitions and games in sports, organized exercise other than sports, and unsupervised PA. The adolescents were asked to report whether they had done specific types of PA during the previous year. They were then asked to report the number of months of each PA per year, the number of sessions of each PA per week, and the duration of a single session of each type of PA separately on weekdays and weekend days. The total amount of each PA was calculated and expressed in hours per day. The amount of total PA was calculated by summing the amount of each PA type and was expressed in hours per day.
Sedentary time included watching TV and videos, using the computer or a tablet, playing video games, using a mobile phone and playing mobile games, listening to music, playing musical instruments, singing, reading, writing, drawing, doing arts and crafts, playing board and card games, cooking and baking, and sitting and lying for a rest. The amount of sedentary time was calculated by summing the amount of each type of sedentary time, weighted by the numbers of weekdays and weekend days, and was expressed in hours per day.
Assessment of body size and composition
Body height and weight were assessed the children having fasted for 12 h. Body height was assessed using a wall-mounted stadiometer and body weight using the InBody® 720 bioelectrical impedance device (Biospace, Seoul, South Korea), with the weight assessment integrated into the system. We computed age- and sex-standardized BMI-SDS using Finnish references [29]. Body fat percentage was measured in the supine position, empty-bladdered and in light clothing by dual-energy X-ray absorptiometry (DXA) using the Lunar DXA device (Lunar Prodigy Advance; GE Medical Systems).
Assessment of other determinants of serum 25-hydroxyvitamin D concentration
Daylight time from sunrise to sunset in Kuopio, Finland, at latitude 62.89°N, was calculated as the average during three months before the blood sampling. The daylight time was provided by the Almanac Office, University of Helsinki. The seasons of blood sampling were determined as winter (December–February), spring (March–May), summer (June–August) and autumn (September–November). Travels to sunny countries within three months before the blood sampling (no, yes), sunscreen use (no, yes), skin color type (four categories according to Fitzpatrick [30] from light to dark), sun preference behavior (avoiding sun, not avoiding sun), race (Caucasian, non-Caucasian), parental education, and household income were assessed using questionnaires completed by the adolescents or their parents. Skin types I–II and III–IV were combined. Parental education was defined as the highest completed or ongoing degree of the parents and was categorized in two classes as occupational education, occupational institute or university of applied sciences, and university. Household income was reported to accuracy of 10 000 € and was categorized as ≤ 60000 €/y or > 60000 €/y.
Measurement of serum 25-hydroxyvitamin D concentration
Venous blood samples were taken after 12-h overnight fasting. Blood was immediately centrifuged and stored at a temperature of −75 °C until biochemical analyses. Serum 25(OH)D concentration was analyzed by a chemiluminescence immunoassay the LIAISON® 25 OH vitamin D TOTAL Assay (DiaSorin, Stillwater, MN) using an automatic LIAISON® XL immunoanalyzer (DiaSorin). The assay range is from 10 to 375 nmol/l. Intra-assay variation was 1.7% at the concentration range of 21–103 nmol/l. Inter-assay variation was from 6.1 to 7.0%. The 25(OH)D analyses were performed in Eastern Finland Laboratory Centre Joint Authority Enterprise, which participates in the vitamin D External Quality Assessment Scheme and met the performance targets of this quality control system in January 2021. The LIAISON® 25 OH vitamin D TOTAL Assay has received the certificate of the vitamin D Standardization-Certification Program [31].
Statistical analyses
Statistical analyses were performed using the IBM SPSS Statistics®, Version 27 (IBM Corp., Armonk, NY, USA). The normality of distributions of the variables was verified visually and by the Kolmogorov–Smirnov test. The T test for independent samples for continuous variables with normal distributions, the Mann–Whitney U test for continuous variables with skewed distributions and the Pearson χ2 test for categorical variables were used to examine differences in the basic characteristics between sexes. We calculated the contribution of each food group to the total intake of vitamin D using the population proportion method as defined by Krebs–Smith and coworkers [32]. Food groups that provided at least 4% of dietary intake of vitamin D were used in the linear regression analysis that was used to investigate the determinants of serum 25(OH)D concentration. Fish consumption was divided into two classes (yes, no) for regression models due to very skewed distribution. Body fat percentage was the strongest correlate of 25(OH)D among variables related to body composition and was used in regression analyses. The variables were first entered one by one into the models and then entered stepwise into the model to study whether they were independently associated with serum 25(OH)D concentration. Risk factors of having serum 25(OH)D concentration < 50 nmol/l were studied using logistic regression analysis adjusted for age and sex. For these analyses, vitamin D supplement use was divided into 0, 0.01–9.99 and ≥ 10 µg/d and other continuous variables were categorized into thirds. Differences and associations with a p value of < 0.05 were considered statistically significant.
Results
Characteristics of children
Boys had higher body weight and height, and lower body fat percentage, were physically more active and less likely to use sunscreen, had higher vitamin D intake from food and higher total vitamin D intake from food and supplements, higher serum 25(OH)D concentration, and a higher proportion of parents having university education than girls (Table 1). Boys consumed more milk products, fat products, meat products, grain products and other food but less fruits and berries than girls (Table 2).
Vitamin D intake from food and supplements
The mean total intake of vitamin D, including food and supplements, was 19.2 µg/d, and the mean intake of vitamin D from food was 9.9 µg/d (Table 1). Altogether, 71.3% of adolescents used supplements with vitamin D. The median of the average intake of vitamin D from supplements for those who used vitamin D supplements was 8.6 µg/d. The adolescents in the intervention group had higher mean (SD) dietary intake of vitamin D than those in the control group (10.5 [5.7] vs. 8.9 [4.8] µg/d, p = 0.025), but there was no difference in the median (IQR) of vitamin D intake from supplements among supplement users in the intervention group and the control group (8.7 [9.3] vs. 8.6 [11.1] µg/d, p = 0.707) or in the mean (SD) of total vitamin D intake (19.6 [12.3] vs. 18.6 [13.2] µg/d, p = 0.595). Of all adolescents, 75% (66.7% of girls, 82.7% of boys) met the Finnish recommendation of 10 µg/d for the total vitamin D intake from food and supplements [7, 13]. Total vitamin D intake was below the Finnish recommendation in 7.8% of the adolescents who used vitamin D supplements and in 69.5% of those who did not use supplements (p < 0.001). Altogether, 48.8% of the adolescents who used supplements met the target of daily vitamin intake of 10 µg/d already from food, whereas 30.5% of the adolescents who did not use supplements met this target (p = 0.016).
Sources of vitamin D
Vitamin D supplements were the most important source of vitamin D, accounting for 48.4% of total vitamin D intake, followed by milk products (24.9%), fat products (16.8%), fish products (5.3%), meat products (2.6%), egg (1.2%) and other food (0.9%). Dietary sources of vitamin D (excluding supplements) are described in Table 3.
Serum 25-hydroxyvitamin D concentration
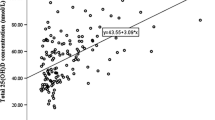
Serum 25(OH)D concentration varied between 16.4 and 153.0 nmol/l. The mean serum 25(OH)D was 62.0 nmol/l (Table 1). There was no difference in mean (SD) serum 25(OH)D concentration between adolescents in the intervention group and those in the control group (62.9 [18.9] vs. 60.6 [18.6], p = 0.325). Distribution of serum 25(OH)D concentration is illustrated in Fig. 1. Serum 25(OH)D was < 50 nmol/l in 29.5% of all adolescents, 31.6% of the girls and 27.7% of the boys and < 30 nmol/l in 1.9% of the adolescents, 1.7% of the girls and 2.0% of the boys. Altogether, 23.3% of the adolescents who used supplements had serum 25(OH)D < 50 nmol/l, whereas 44.7% of those who did not use them had serum 25(OH)D < 50 nmol/l (p = 0.001).
Determinants of serum 25-hydroxyvitamin D concentration
In all adolescents, male sex, higher parental education, higher household income, lower body fat percentage, higher levels of PA, lower levels of sedentary time, longer average daylight time, travels to sunny countries, higher consumption of milk products, fat products, and meat products, and higher intake of vitamin D from supplements were associated with higher serum 25(OH)D without adjustments (Table 1, Model 1). Higher intake of vitamin D from supplements, higher consumption of milk products and meat products, travels to sunny countries, and higher daylight time were associated with higher serum 25(OH)D when all variables listed in Table 4 were entered simultaneously in the stepwise model (Table 4, Model 2).
Among girls, lower body fat percentage, higher consumption of milk products, and higher intake of vitamin D from supplements were associated with higher serum 25(OH)D without adjustments (Table 1, Model 1). Higher vitamin D intake from supplements, higher consumption of milk products, travels to sunny countries, and an older age were associated with higher serum 25(OH)D in girls when all variables listed in Table 4 were entered simultaneously in the stepwise model (Table 4, Model 2).
Among boys, lower body fat percentage, higher levels of PA, lower levels of sedentary time, higher daylight time and travels to sunny countries, sunscreen use, higher consumption of milk products and meat products, and higher intake of vitamin D from supplements were associated with higher serum 25(OH)D without adjustments (Table 4, Model 1). Higher intake of vitamin D from supplements, higher consumption of milk products and meat products, travels to sunny countries and longer average daylight time were associated with higher serum 25(OH)D in boys when all variables listed in Table 4 were entered simultaneously in the stepwise model (Table 4, Model 2).
A higher dietary intake of vitamin D was associated with a higher serum 25(OH)D concentration in all adolescents (β = 0.289, p < 0.001), and in boys (β = 0.288, p = 0.002), but the association was not statistically significant in girls (β = 0.174, p = 0.072), without adjustments.
Risk factors of having serum 25-hydroxyvitamin D concentration below 50 nmol/l
Adolescents who used vitamin D supplements at least 10 µg/d had 81% lower and those who used vitamin D supplements < 10 µg/d had 57% lower odds of having serum 25(OH)D concentration < 50 nmol/l than those who did not use supplements at all, adjusted for age and sex (Table 5). Adolescents who consumed milk at least 550 g/d (highest third) had 77% lower odds of having serum 25(OH)D < 50 nmol/l than those who consumed milk less than 250 g/d (lowest third). Adolescents who consumed meat at least 151 g/d (highest third) had 65% lower odds of having serum 25(OH)D < 50 nmol/l than those who consumed meat less than 92.4 g/d (lowest third). Adolescents with average daylight time of over 14.8 h/d during three months before blood sampling (highest third) had 72% lower and those with average daylight time of 10.0–14.8 h/d (middle third) had 63% lower odds of having serum 25(OH)D < 50 nmol/l than those with daylight time less than 10 h/d (lowest third). Adolescents whose blood samples were collected in summer had 79% lower, those whose blood samples were collected in spring had 58% lower, and those whose blood samples were collected in autumn had 55% lower odds of having serum 25(OH)D < 50 nmol/l than those whose blood samples were collected in winter. Adolescents who had travelled to sunny countries during three months before blood sampling had 76% lower odds of having serum 25(OH)D < 50 nmol/l than those without such travelling, and those who used sunscreen had 66% lower odds of having serum 25(OH)D < 50 nmol/l than those who did not use sunscreen. Adolescents whose parents had university education had 46% lower odds of having serum 25(OH)D < 50 nmol/l than those whose parents had lower education, and those whose parents reported household income ≥ 60000 €/y had 70% lower odds of having serum 25(OH)D < 50 nmol/l than those whose parents had lower income.
Discussion
The results from our study in a population sample of Finnish adolescents showed that 25% did not meet the recommendation for total vitamin D intake from food and supplements of 10 µg/d [7, 13], 29% did not use vitamin D supplements, and 30% had serum 25(OH)D concentration < 50 nmol/l. Vitamin D intake from supplements and milk consumption were the most important determinants of vitamin D status, followed by consumption of meat products, travels to sunny countries, and average daylight time during three months before blood sampling. Milk and vegetable oil-based spreads, which are mostly fortified with vitamin D in Finland, were the main dietary sources of vitamin D. Using vitamin D supplements, consuming milk over 550 g/d, consuming meat and meat products over 151 g/d, having average daylight time over 10 h/d or travelling to sunny countries during three months before blood sampling, blood sampling in other seasons than winter, using sunscreen, having parents with higher education, and having higher household income were associated with lower odds of having serum 25(OH)D < 50 nmol/l.
The proportion of adolescents with serum 25(OH)D < 50 nmol/l in our Finnish study sample of 30% was lower than in other European study populations of school-age children and adolescents using standardized 25(OH)D concentrations [20], lower than in a large Swedish study population in adolescents [33] and almost same as in a Canadian study population of children and adolescents [21]. Only 0.8% of the adolescents in our study had serum 25(OH)D < 25 nmol/l and 1.9% had serum 25(OH)D < 30 nmol/l. However, the mean serum 25(OH)D was lower and the proportion of adolescents with serum 25(OH)D < 50 nmol/l was higher than among Finnish adults in 2011 [15] and in 2017 [17] and among 10-year-old Finnish children in 2013 [19] after the increase in the recommended vitamin D intake, supplementation, and fortification in Finland in 2010–2014 [7, 12, 13]. To the best of our knowledge, there are no previous data on serum 25(OH)D concentrations in Finnish adolescents after the updated national recommendations on vitamin D. The present data support the evidence that low vitamin D intake and vitamin D insufficiency remain common problems among children and adolescents in Nordic countries and elsewhere in Europe [3, 33, 34].
The average vitamin D intake among adolescents in the current study was 9.9 µg/d from food and 19.2 µg/d in total from food and supplements, which is well above the recommended 10 µg/d [7, 13], only slightly lower than in Finnish children 3–6 years of age [18], and higher than in school-age children and adolescents in other Nordic countries [34, 35]. Finnish children and adolescents 2–18 years of age are recommended to use vitamin D supplements 7.5 µg daily [13], and 71% of the adolescents in our study reported using supplements, even though many of them did not use supplements daily year-round. Vitamin D supplement use among adolescents was more common than in other Nordic countries [34]. However, 25% of all adolescents and up to 70% of those who did not use supplements did not meet the recommendation of total vitamin D intake 10 µg/d. In the most recent national FinDiet survey among Finnish adults in 2017, the mean vitamin D intake from food and supplements was higher than in adolescents of our study [17, 36].
The use of vitamin D supplements has been associated with higher serum 25(OH)D [14, 21, 37, 38] and a lower risk of having vitamin D deficiency in children and adolescents [39]. The use of vitamin D supplement has also been reported to reduce seasonal variation in serum 25(OH)D [40]. Vitamin D intake from supplements was the strongest determinant of serum 25(OH)D in the current study, and adolescents not using vitamin D supplements had higher odds of having low serum 25(OH)D than those using these supplements. The adolescents in the intervention group, the intervention having initiated eight years earlier in childhood, being based on the Finnish nutrition recommendations, and having resulted in a higher vitamin D intake than average in a Finnish diet [23, 36], had higher dietary intake of vitamin D than those in the control group, but there was no difference in serum 25(OH)D levels between the groups. Interestingly, a higher proportion of supplement users than non-users met the recommendation of total daily vitamin D intake 10 µg already from food without supplements, which suggests that those who eat healthier also follow the recommendations on supplement use better.
Fortified milk was the main dietary source of vitamin D, followed by fortified vegetable oil-based spreads, in adolescents participating in our study. They consumed more milk and less vegetable oil-based spreads and fish than adults in the FinDiet survey [36], which explains that distribution of dietary sources differed from that of adults [17]. Milk consumption was also the most important dietary determinant of serum 25(OH)D after the use of vitamin D supplements. Meat consumption was associated with serum 25(OH)D in boys but not in girls whereas consumption of fat products was not related to serum 25(OH)D in either sex even though fat products were a more important source of vitamin D. One explanation for this may be that meat consumption was high, boys consumed more meat than girls, and 25(OH)D that is found in meat has been suggested to have higher biopotency to increase serum 25(OH)D than D3 and especially D2, which are added in fortified fat products [7, 41]. Meat consumption was higher than recommended [13] especially in the boys. Because the production of meat and milk products has great environmental effects, alternative products including vitamin D are needed. However, the consumption of plant-based products fortified with vitamin D were too low to contribute to vitamin D intake in the current study. Up to 45% of the adolescents did not report consuming fish, and vitamin D intake from the natural sources of vitamin D such as fish and eggs [7] was low, which explains that they were not associated with serum 25(OH)D. The fish species eaten was reported in food records, but due to the low consumption of fish, we could not separate the consumption of fish species with higher vitamin D content in the linear regression analyses. Therefore, it is possible that the consumption of fish species containing most vitamin D [27] may have been too low to contribute 25(OH)D.
In Finland, milk and other dairy products are commonly used, which is one of the reasons why fortifying milk with vitamin D has been an effective strategy to increase vitamin D intake and serum 25(OH)D at the population level [15, 16]. Fortified milk products have been an important source of vitamin D and a major correlate of serum 25(OH)D also in other countries with national vitamin D fortification policy, such as in Canada and the USA, whereas their role has been smaller in countries where fortification is less systematic, such as in Sweden and Norway [42]. A recent national survey in Swedish adolescents found that adolescents with serum 25(OH)D < 30 nmol/l consumed less fortified products, even though the study was performed before implementing higher fortification policy in Sweden in 2018 [33]. We found that low serum 25(OH)D was more common and also dietary intake of vitamin D was lower in adolescents who did not use supplements than in those who used them. Current fortification policy seems effective in raising serum 25(OH)D among those who consume fortified products [15]. However, it is possible that a low-level fortification of several products may help increase vitamin D intake of the risk group that does not meet the recommendation of dietary vitamin D intake nor follow the recommendation to use supplements.
Girls had lower serum 25(OH)D than boys, which has been shown also in other studies among children and adolescents [14, 37, 43, 44]. Moreover, girls had lower vitamin D intake from food, which partly explains the sex difference. Another reason for the sex difference could be that girls had higher body fat percentage than boys. Obesity has been associated with decreased serum 25(OH)D in children and adolescents [21, 37, 44, 45], which may be due to storing vitamin D in adipose tissue [46], attenuated release of D3 from the skin into the circulation [47], impaired diet, or limited time spent outdoors. In the current study, higher body fat percentage was associated with lower serum 25(OH)D, but the association was explained by other factors. Boys had more PA, which may be associated with time spent outdoors that increases cutaneous synthesis of vitamin D and partly explain their higher serum 25(OH)D compared with girls. Higher levels of PA have been associated with higher serum 25(OH)D in children and adolescents [38, 44, 45]. Higher screen time or sedentary time has also been associated with a higher risk of vitamin D deficiency in children [37, 48] an in adolescents [33]. We found that higher levels of PA and lower levels of sedentary time were associated with higher serum 25(OH)D, but these associations were largely explained by other determinants.
Higher parental education and household income had weak positive associations with serum 25(OH)D in adolescents participating in our study. The adolescents whose parents had higher education or higher household income also had lower odds of having serum 25(OH)D < 50 nmol/l. In line with the findings of previous studies [21, 43, 48], the results of our study suggest that a higher socioeconomic position may be associated with a better vitamin D status.
Darker skin is known to produce less vitamin D than light skin [49], and adolescents with dark skin or a non-Caucasian race have been found to be at increased risk of low serum 25(OH)D [21, 44]. However, skin type was not associated with serum 25(OH)D in the current study in mostly Caucasian adolescents. Because the number of adolescents with very light and dark skin was very low, we combined skin types I–II and III–IV in the analyses, which may explain why an association between skin type and serum 25(OH)D was not found.
Sunlight has been stated to be the main determinant of vitamin D status at the global level, and several studies have shown seasonal variation in serum 25(OH)D with lowest concentrations observed in winter or early spring [14, 21, 40, 43, 44]. We found that longer average daylight time during three months before blood sampling was associated with higher serum 25(OH)D and that adolescents who had average daylight time < 10 h/d during this time period or were studied in winter had increased odds of having serum 25(OH)D < 50 nmol/l. Longer average daylight time was associated with higher serum 25(OH)D in boys, but not in girls. One reason for this may be a larger variation in exposure to sunlight and vitamin D production in the skin among boys part of whom spend a lot of time outdoors because of their physically active lifestyle. Travels to sunny countries were also associated with higher serum 25(OH)D. This association is also likely due to more time spent outdoors and higher cutaneous synthesis of vitamin D in lower latitudes than in Finland. Sunscreen use may reduce vitamin D production in the skin [49]. However, adolescents who used sunscreen had lower odds of having serum 25(OH)D < 50 nmol/l than those not using it, and such association of sunscreen in linear models was seen only in boys. One reason for this might be that boys who used sunscreen spent more time outdoors than girls using it. Another explanation for the sex difference could be that girls used sunscreen more frequently or in larger amounts than boys and that sunscreen use may have been sufficient to reduce the cutaneous synthesis of vitamin D induced by sunlight in girls but not in boys.
The strengths of this study include a population-based sample of adolescents whose vitamin D status and its determinants have been studied scarcely, the assessment of dietary vitamin D intake and other dietary factors using food records, the assessment of several other relevant determinants of serum 25(OH)D, and the measurement of serum 25(OH)D by an assay certified in Vitamin D Standardization-Certification Program [31]. High variation in daylight time across seasons due to high latitude allow us to study the association between daylight time and serum 25(OH)D, as sunlight is inadequate for vitamin D synthesis in the skin approximately half of the year. A weakness of this study is the assessment of vitamin D intake from supplements by a questionnaire, which may have underestimated or overestimated the use of vitamin D supplements. The number of adolescents who reported to avoid sun was also low, and we therefore were not able to analyze the association of sun preference behavior with serum 25(OH)D. We did not collect data on time spent outdoors that could only indirectly be estimated by the levels of PA that is partly performed outdoors. More health conscious and motivated adolescents may have participated in the study until the 8-year examinations compared with the drop-outs, which is a common phenomenon in studies with a long follow-up. However, the participants of this study did not differ in age, BMI-SDS, vitamin D intake from food or supplements, or the distribution of sex or study groups at baseline from those who dropped out. A large national survey is required to confirm if the results are generalizable nationwide as it is possible that there may be some local differences in intake, sources, and determinants of vitamin D. Finally, as cross-sectional data are used, causality of the associations cannot be proved.
Conclusions
This study shows that 25–30% of the Finnish adolescents did not meet the recommendation for vitamin D intake from food and supplements, and 30% of the adolescents had serum 25(OH)D concentration < 50 nmol/l. Vitamin D supplement use and milk consumption were the main sources of vitamin D and the most important determinants of serum 25(OH)D. These findings emphasize that adolescents from Northern latitudes who do not use vitamin D supplements and consume fortified milk products are at increased risk of insufficient serum 25(OH)D concentrations.
Data availability
The PANIC Study is ongoing and therefore the data are not fully anonymized and, thus, not openly available. Part of the data can be shared by request.
Abbreviations
- 25(OH)D:
-
25-Hydroxyvitamin D
- BMI-SDS:
-
Body mass index standard deviation score
- DXA:
-
Dual-energy X-ray absorptiometry
- PA:
-
Physical activity
- PANIC Study:
-
Physical Activity and Nutrition in Children Study
References
Munns CF, Shaw N, Kiely M et al (2016) Global consensus recommendations on prevention and management of nutritional rickets. Horm Res Paediatr 85:83–106. https://doi.org/10.1159/000443136
Lamberg-Allardt C, Brustad M, Meyer HE, Steingrimsdottir L (2013) Vitamin D-a systematic literature review for the the 5th edition of the Nordic Nutrition Recommendations. Food Nutr Res. https://doi.org/10.3402/fnr.v57i0.22671
Lips P, Cashman KD, Lamberg-Allardt C et al (2019) Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol 180:P23–P54. https://doi.org/10.1530/EJE-18-0736
Harel Z, Cromer B, DiVasta AD et al (2013) Recommended vitamin D intake and management of low vitamin D status in adolescents: a position statement of the society for adolescent health and medicine. J Adolesc Heal 52:801–803. https://doi.org/10.1016/j.jadohealth.2013.03.022
Holick MF, Binkley NC, Bischoff-Ferrari HA et al (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930. https://doi.org/10.1210/jc.2011-0385
Institute of Medicine (2011) Dietary reference intakes for calcium and vitamin D. National Academies Press, Washington, DC
Nordic Council of Ministers (2014) Nordic nutrition recommendations. Integrating nutrition and physical activity, 5th edn. Nordic Council of Ministers, Copenhagen
Scientific Advisory Committee on Nutrition (2016) Vitamin D and health. https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition. (Accessed 7 Apr 2022)
Bresson JL, Burlingame B, Dean T et al (2016) Dietary reference values for vitamin D. EFSA J. https://doi.org/10.2903/j.efsa.2016.4547
Vitamin D Standardization program (VDSP), Binkley N, Sempos CT (2014) Standardizing vitamin D assays: the way forward. J Bone Miner Res 29:1709–1714. https://doi.org/10.1002/jbmr.2252
Kiely M, Cashman KD (2018) Summary outcomes of the ODIN project on food fortification for vitamin D deficiency prevention. Int J Environ Res Public Health 15:2342. https://doi.org/10.3390/ijerph15112342
National Nutrition Council (2010) VRN D-vitamiinityöryhmän raportti huhtikuu 2010. (Report of vitamin D working group, in Finnish.) Available at: https://www.ruokavirasto.fi/globalassets/teemat/terveytta-edistava-ruokavalio/vrn/d-vitamiiniraportti2010.pdf. Accessed 29 Aug 2022
National Nutrition Council (2014) Finnish nutrition recommendations 2014. Juvenes Oy, Helsinki
Soininen S, Eloranta A-M, Lindi V et al (2016) Determinants of serum 25-hydroxyvitamin D concentration in Finnish children: the physical activity and nutrition in children (PANIC) study. Br J Nutr 25:1–12. https://doi.org/10.1017/S0007114515005292
Jääskeläinen T, Itkonen ST, Lundqvist A et al (2017) The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data. Am J Clin Nutr 105:1512–1520. https://doi.org/10.3945/ajcn.116.151415
Raulio S, Erlund I, Männistö S et al (2017) Successful nutrition policy: improvement of vitamin D intake and status in Finnish adults over the last decade. Eur J Public Health 27:268–273. https://doi.org/10.1093/eurpub/ckw154
Raulio S, Tapanainen H, Valsta L et al (2021) FinRavinto 2017-tuloksia: D-vitamiinin saanti ja seerumipitoisuus aikuisilla. Suom Lääkäril–Finlands läkartidning 76:2578–2582
Skaffari E, Korkalo L, Vepsäläinen H, et al (2019) Päiväkoti-ikäisten lasten ruokavalio -raportti. In: Helsingin Yliop. https://dagis.fi/raportit-muut-julkaisut/. (Accessed 7 Apr 2022)
Rosendahl J, Fogelholm M, Pelkonen A et al (2017) A history of cow’s milk allergy is associated with lower vitamin D status in schoolchildren. Horm Res Paediatr 88:244–250. https://doi.org/10.1159/000478779
Cashman KD, Dowling KG, Škrabáková Z et al (2016) Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr 103:1033–1044. https://doi.org/10.3945/ajcn.115.120873
Munasinghe LL, Yuan Y, Willows ND et al (2017) Vitamin D deficiency and sufficiency among Canadian children residing at high latitude following the revision of the RDA of vitamin D intake in 2010. Br J Nutr 117:457–465. https://doi.org/10.1017/S0007114517000320
Lakka TA, Lintu N, Väistö J et al (2020) A 2 year physical activity and dietary intervention attenuates the increase in insulin resistance in a general population of children: the PANIC Study. Diabetologia 63:2270–2281. https://doi.org/10.1007/s00125-020-05250-0
Sallinen T, Viitasalo A, Lintu N et al (2022) The effects of an 8-year individualised lifestyle intervention on food consumption and nutrient intake from childhood to adolescence: the PANIC Study. J Nutr Sci 11:e40. https://doi.org/10.1017/jns.2022.13
Ministry of Social Affairs and Health (2005) Recommendations for physical activity in early childhood education. Handbooks of the Ministry of Social Affairs and Health, Helsinki (In Finnish)
National Nutrition Council (2005) Finnish nutrition recommendations. Edita Prima Oy, Helsinki
Rastas M, Seppänen R, Knuts L et al (1997) Nutrient composition of foods. Publications of the Social Insurance Institution, Helsinki, Finland
National Institute for Health and Welfare, Nutrition Unit. In: Fineli. Finnish food Compos. database. https://fineli.fi/fineli/fi/index. (Accessed 7 Apr 2022)
Ministry of Agriculture and Forestry of Finland Maa-ja metsätalousministeriön asetus rasvattoman homogenoidun maidon D-vitaminoinnista. Available online: http://www.finlex.fi/fi/laki/alkup/2016/20160754. (In Finnish, Accessed 29 Aug 2022)
Saari A, Sankilampi U, Hannila M-L-L et al (2011) New Finnish growth references for children and adolescents aged 0 to 20 years: length/height-for-age, weight-for-length/height, and body mass index-for-age. Ann Med 43:235–248. https://doi.org/10.3109/07853890.2010.515603
Fitzpatrick TB (1988) The validity and practicality of sunreactive skin types I through VI. Arch Dermatol 124:869–871. https://doi.org/10.1001/archderm.124.6.869
Vitamin D Standardization-Certification Program (VDSCP) | CDC. https://www.cdc.gov/labstandards/vdscp.html. (Accessed 7 Apr 2022)
Krebs-Smith SM, Kott PS, Guenther PM (1989) Mean proportion and population proportion: two answers to the same question? J Am Diet Assoc 89:671–676
Warensjö Lemming E, Petrelius Sipinen J, Nyberg G et al (2022) Vitamin D status and associations with diet, objectively measured physical activity patterns and background characteristics among adolescents in a representative national cross-sectional survey. Public Health Nutr. https://doi.org/10.1017/S1368980022000222
Itkonen ST, Andersen R, Björk AK et al (2021) Vitamin D status and current policies to achieve adequate vitamin D intake in the Nordic countries. Scand J Public Health 49:616–627. https://doi.org/10.1177/1403494819896878
Warensjö Lemming E, Moraeus L, Petrelius Sipinen J, Lindroos AK (2013) Riksmaten ungdom 2016–2017. Närningsintag och närningsstatus bland ungdomar i Sverige (in Swedish with English abstract)
Valsta L, Kaartinen N, Tapanainen H et al (2018) Ravitsemus Suomessa–nutrition in Finland. THL, Helsinki
Kumar J, Muntner P, Kaskel FJ et al (2009) Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 124:e362–e370. https://doi.org/10.1542/peds.2009-0051
Petersen RA, Damsgaard CT, Dalskov SM et al (2016) Vitamin D status and its determinants during autumn in children at northern latitudes: a cross-sectional analysis from the optimal well-being, development and health for Danish children through a healthy New Nordic Diet (OPUS) School Meal Study. Br J Nutr 115:239–250. https://doi.org/10.1017/S000711451500433X
Absoud M, Cummins C, Lim MJ et al (2011) Prevalence and predictors of vitamin D insufficiency in children: a great britain population based study. PLoS ONE. https://doi.org/10.1371/journal.pone.0022179
Hansen L, Tjønneland A, Køster B et al (2018) Vitamin D status and seasonal variation among danish children and adults: a descriptive study. Nutrients. https://doi.org/10.3390/nu10111801
Taylor CL, Patterson KY, Roseland JM et al (2014) Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. J Nutr 144:654–659. https://doi.org/10.3945/jn.113.189811
Itkonen S, Erkkola M, Lamberg-Allardt C (2018) Vitamin D fortification of fluid milk products and their contribution to vitamin D intake and vitamin D status in observational studies—a review. Nutrients 10:1054. https://doi.org/10.3390/nu10081054
Tolppanen AM, Fraser A, Fraser WD, Lawlor DA (2012) Risk factors for variation in 25-hydroxyvitamin D(3) and D(2) concentrations and vitamin D deficiency in children. J Clin Endocrinol Metab 97:1202–1210. https://doi.org/10.1210/jc.2011-2516
Dong Y, Pollock N, Stallmann-Jorgensen IS et al (2010) Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics 125:1104–1111. https://doi.org/10.1542/peds.2009-2055
Vierucci F, Del Pistoia M, Fanos M et al (2014) Prevalence of hypovitaminosis D and predictors of vitamin D status in Italian healthy adolescents. Ital J Pediatr. https://doi.org/10.1186/1824-7288-40-54
Abbas MA (2017) Physiological functions of vitamin D in adipose tissue. J Steroid Biochem Mol Biol 165:369–381. https://doi.org/10.1016/j.jsbmb.2016.08.004
Wortsman J, Matsuoka LY, Chen TC et al (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–693. https://doi.org/10.1093/ajcn/72.3.690
Voortman T, van den Hooven EH, Heijboer AC et al (2015) Vitamin D deficiency in school-age children is associated with sociodemographic and lifestyle factors. J Nutr 145:791–798. https://doi.org/10.3945/jn.114.208280
Engelsen O (2010) The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients 2:482–495. https://doi.org/10.3390/nu2050482
Acknowledgements
We are grateful to all participants of the PANIC Study. We also thank the personnel of the PANIC Study for their invaluable contribution.
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital. The PANIC Study has financially been supported by Ministry of Education and Culture of Finland, Ministry of Social Affairs and Health of Finland, Research Committee of the Kuopio University Hospital Catchment Area (State Research Funding), Finnish Innovation Fund Sitra, Social Insurance Institution of Finland, Finnish Cultural Foundation, Foundation for Paediatric Research, Diabetes Research Foundation in Finland, Finnish Foundation for Cardiovascular Research, Juho Vainio Foundation, Paavo Nurmi Foundation, Yrjö Jahnsson Foundation, and the City of Kuopio. Sonja Soininen was personally supported by Research Committee of the Kuopio University Hospital Catchment Area (State Research Funding), Finnish Cultural Foundation, Foundation for Paediatric Research, Finnish Medical Society Duodecim, and Orion Research Foundation sr.
Author information
Authors and Affiliations
Contributions
SS wrote the draft of the manuscript, conducted the statistical analyses, and received funding. TAL designed the study and received funding. SS, AME, US, and TAL participated in the collection of the data, formulated research question, interpreted the findings, contributed to the critical revision of the manuscript, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical approval
The Research Ethics Committee of the Hospital District of Northern Savo approved the study protocol in 2006 (Statement 69/2006) and in 2015 (Statement 422/2015). The PANIC Study has been carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008.
Consent to participate
At baseline, the caregivers gave their written informed consent, and the children provided their assent to participation. At 8-year follow-up, both caregivers and adolescents gave their written informed consent.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soininen, S., Eloranta, AM., Schwab, U. et al. Sources of vitamin D and determinants of serum 25-hydroxyvitamin D in Finnish adolescents. Eur J Nutr 62, 1011–1025 (2023). https://doi.org/10.1007/s00394-022-03039-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03039-y