Abstract
Purpose
Research suggests that diet influences cognitive function and the risk for neurodegenerative disease. The present study aimed to determine whether a recently developed diet score, based on recommendations for dietary priorities for cardio metabolic health, was associated with fluid intelligence, and whether these associations were modified by individual genetic disposition.
Methods
This research has been conducted using the UK Biobank Resource. Analyses were performed using self-report data on diet and the results for the verbal-numerical reasoning test of fluid intelligence of 104,895 individuals (46% male: mean age at recruitment 57.1 years (range 40–70)). For each participant, a diet score and a polygenic score (PGS) were constructed, which evaluated predefined cut-offs for the intake of fruit, vegetables, fish, processed meat, unprocessed meat, whole grain, and refined grain, and ranged from 0 (unfavorable) to 7 (favorable). To investigate whether the diet score was associated with fluid intelligence, and whether the association was modified by PGS, linear regression analyses were performed.
Results
The average diet score was 3.9 (SD 1.4). After adjustment for selected confounders, a positive association was found between baseline fluid intelligence and PGS (P < 0.001). No association was found between baseline fluid intelligence and diet score (P = 0.601), even after stratification for PGS, or in participants with longitudinal data available (n = 9,482).
Conclusion
In this middle-aged cohort, no evidence was found for an association between the investigated diet score and either baseline or longitudinal fluid intelligence. However, as in previous reports, fluid intelligence was strongly associated with a PGS for general cognitive function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elucidating the role of dietary intake in cognitive function, cognitive decline, and neurodegenerative disease development is important in terms of disease prevention. Research has shown that nutrition influences many of the molecular mechanisms that underlie cognitive function, such as neurogenesis, synaptic plasticity, and neuronal connectivity [1,2,3]. However, studies of the association between cognitive function, cognitive decline, and/or the risk of dementia and dietary patterns, such as the Mediterranean, Nordic, and DASH (Dietary Approaches to Stop Hypertension) diets, have generated inconclusive results [4,5,6,7,8,9,10,11]. Only the Mediterranean-Dietary Approaches to Stop Hypertension Diet Intervention for Neurodegenerative Delay (MIND) diet has consistently shown beneficial associations in terms of the risk of dementia and cognitive decline [12, 13]. However, a more consistent research finding is that dietary habits influence cardiometabolic risk factors, including blood pressure, glucose-insulin homeostasis, lipoprotein concentrations and function, and inflammation [14]. Interestingly, several studies have demonstrated that a higher risk of cognitive impairment in later life is associated with cardiovascular risk factors during middle age [15,16,17,18]. Based on recommendations for dietary priorities for cardio metabolic health [14], Lourida et al. constructed a diet score as one component of a lifestyle score [19]. Analyses based on the population-based UK Biobank showed that participants with an unfavorable lifestyle had a higher risk of dementia over an 8 year period of follow-up.
Cognitive function incorporates multiple domains and abilities. One cognitive function domain is fluid intelligence, which refers to the capacity for reasoning and novel problem-solving [20]. Research has shown that declines in the ability to live and function independently as a person correlates highly with decline in fluid intelligence [21, 22]. Hence, an improved understanding of the determinants of fluid intelligence is warranted [19, 23].
As with dementia, cognitive functions also show a substantial degree of heritability [24]. Heritability estimates for cognitive function range between 12 and 25% [25,26,27], and recent genome-wide association studies (GWAS) of general cognitive function have identified more than 140 associated loci [24,25,26,27].
To our knowledge, no study to date has investigated whether: (1) the diet score of Lourida et al. [19] is associated with fluid intelligence; or (2) genetic disposition for cognitive function alters the association between diet and fluid intelligence. Therefore, the aim of the present study was to determine whether the “healthy diet” score [19] was associated with baseline fluid intelligence and change in fluid intelligence over time in the UK Biobank cohort. To test whether this association was influenced by individual genetic disposition, a polygenic score (PGS) approach was applied, which takes into consideration the polygenic nature of complex traits [28]. A score comprising of a set of single-nucleotide polymorphisms (SNPs) associated with general cognitive function [26] was used.
Research design and methods
The UK Biobank study
This research has been conducted using the UK Biobank Resource. Individual-level data for the present analyses were drawn from the UK Biobank project under application number 31615 “Genetic factors as a biological link between food intake and cognition”. UK Biobank was established to allow detailed longitudinal investigations of the genetic and nongenetic determinants of the diseases of middle and old age [29, 30]. The UK Biobank cohort comprises > 500,000 participants aged 40–69 years at the time of recruitment 55% female). All participants were recruited from the general population. Recruitment invitations were mailed to 9 million individuals, whose contact details were obtained from National Health Service central registers [29].
The ethical approval process for the UK Biobank study is described elsewhere [31].
Baseline assessments were conducted between 2006 and 2010 at a total of 22 assessment centers across the United Kingdom [32]. Here, participants completed a self-report touch-screen questionnaire comprising items on sociodemographic characteristics, general health, medical history, and dietary intake and other lifestyle exposures. During the baseline visit, first, the touch-screen questionnaire was administrated, which was immediately followed by the cognitive function tests [33]. Follow-up examinations commenced in 2012. To date, these have comprised: (1) the first repeat assessment visit (2012–13), (2) the first imaging visit (2014 +), and (3) the first repeat imaging visit (2019 +). The touch-screen questionnaire and other resources can be found on the UK Biobank webpage [32]. Dietary data were assessed in a short touch-screen questionnaire data, which included information on the frequency of the consumption of 29 main foods and food items over the preceding year. At baseline, cognitive functioning was assessed at the assessment using a 15 min self-administered computerized battery, which was developed specifically for the UK Biobank study to enable population-scale cognitive testing that could be administered without researcher supervision [34, 35].
Several of the cognitive function tests administered at the baseline visit were later re-implemented as web-based questionnaires [36]. Hence, from 2014, participants were invited to complete these online tests at home rather than at an assessment center [34]. Fluid intelligence was assessed using the Verbal-Numerical Reasoning (VNR) test [37]. The VNR-test comprises 13 multiple-choice questions that assess verbal and numerical problem-solving. The VNR-test score indicates the number of correct responses achieved within 2 min (range 0–13).
At the baseline UK Biobank assessments, data on lifestyle, including dietary intake, and genotype were obtained for a total of 501,599 and 500,000 participants, respectively [38]. Baseline VNR-test data were available for n = 165,456 participants [39]. A total of 13,470 participants completed the VNR-test at baseline and at a minimum of one follow-up visit.
Present study population
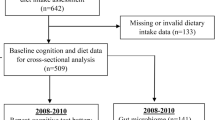
The present analyses were performed using data from 104,895 UK Biobank participants (Fig. 1). Inclusion criteria were the availability of data: on (1) dietary intake, (2) fluid intelligence, and (3) genetic factors. Exclusion criteria were: (1) self-reported ancestry other than “British”, “White”, “Irish”, or “any other white background”; (2) close kinship (third degree or closer relationship with an already recruited participant); (3) missing genetic data; (4) failed quality control (QC) of the genetic data; and (5) missing data on covariates. The analysis of change in fluid intelligence between baseline and the respective follow-up examination/s was performed in participants for whom data from any of the follow-up visits (2012–13; 2015 + , and/or 2019 +) were available (n = 9482 participants).
Diet score
To assess whether dietary patterns were associated with fluid intelligence, a recently developed diet score of Lourida et al. [19] was used. The diet score incorporates seven components (i.e., fruits, vegetables, fish, processed meats, unprocessed red meats, whole grain, and refined grain). For each participant, the diet score was calculated by summing the points for each of the seven food components (range 0–7 points). In accordance with the predefined cut-offs of Lourida et al. [19], fulfillment was scored with 1 point, and non-fulfillment with 0 points (Table 1). A higher score indicates a more favorable diet. For the purposes of the present analyses, three diet score categories were defined: low (0–1 point), intermediate (2–5 points), and high (6–7 points) category.
Genetic data
The imputed (reference: Haplotype Reference Consortium [40]) genetic data were downloaded from the server of UK Biobank [38]. QC was performed for the genetic data of all 104,895 participants included in the present study. Here of all available 93,095,623 genetic markers, all variants with an imputation quality of less than 0.6 were removed (n = 90,078,127). Moreover, SNPs with missing genotype information > 0.03 (n = 187,280), when deviating from Hardy–Weinberg equilibrium (HWE > 0.000001; n = 13,386) or if they were rare variants (minor allele frequency (MAF) < 0.01; n = 1,919,230) were removed as well. This led to a total number of 897,600 genetic variants available in 104,895 participants that were used to construct the PGS. The PGS was constructed by first, clumping the SNPs to capture those that have the lowest p values (based on the GWAS of Davies et al.[26]) in a linkage disequilibrium block (r2 = 0.2, range 1000 kb).
Polygenic score for general cognitive function
Individual genetic disposition for cognitive function was assessed using a PGS approach [28]. For each participant, a PGS was calculated based on common variants discovered in a previous GWAS of general cognitive function in individuals of European ancestry [26]. The PGS was calculated by summing the effect size-weighted number of associated alleles for each participant. For analysis, we used the PGS including all SNPs with p values < 0.2 based on visual inspection of the r-squared value of the crude VNR-score–PGS association. Finally, for each participant, the PGS was z-standardized, and then categorized according to genetic disposition for a high score on the fluid intelligence test using the categories low (lowest quintile), intermediate (quintile 2–4), and high (highest quintile).
Statistical analysis
The baseline characteristics of the study population are presented using means and standard deviations (SD) for continuous variables, and counts and percentages for categorical variables.
Linear regression models were used to test the association between fluid intelligence (i.e., results of the VNR-test) and both the diet score and the PGS. Results obtained from linear regression models are presented in terms of coefficient estimates (beta) with 95% confidence intervals (CI). To adjust the multiple linear regression models, four confounders were considered: age, sex, educational status, and socio economic status (SES). Educational status was categorized as: higher (college or university degree/other professional qualifications e.g., nursing, teaching); upper secondary (A levels/AS levels or equivalent); lower secondary (O levels/ General Certificate of Secondary Educations or equivalent/Certificate of Secondary Education or equivalent); vocational (National Vocational Qualifications or Higher National Diploma or Higher National Certificate or equivalent); or other. SES was assessed using the Townsend deprivation index, which combines information on social class, employment, car ownership, and housing [41]. In accordance with the calculated quintile of the Townsend deprivation index, SES was categorized as low (quintile 1), intermediate (quintiles 2–4), or high (quintile 5). First, univariable regression models were used. Second, all models were adjusted for age, sex, educational status, and the Townsend deprivation index. In addition, models including the PGS were adjusted for the first 20 principal components (PC) of ancestry. To present a measure for effect size, Cohen’s f2 was calculated. As a rule of thumb, f2 = 0.02 indicates a small, f2 = 0.15 a medium, and f2 = 0.35 a large effect [42]. In addition, the analyses were repeated using the continuous diet score and the continuous PGS, rather than the categorized diet score and categorized PGS. Furthermore, each of the seven dietary components was analyzed separately in univariate and adjusted linear regression models, as described above. Additionally, in participants for whom longitudinal data were available, change in fluid intelligence between baseline and the respective follow-up examination/s was analyzed by linear mixed models, with the participant ID being considered as a random effect to account for intra individual dependencies. The linear mixed models were adjusted for the baseline results of the VNR-test, age, sex, educational status, and SES. Analyses were performed to test whether individual genetic disposition modifieds the association between the diet score and performance in the VNR-test by introducing an interaction term (diet score × PGS) into the model, and analyses were stratified according to PGS category. A post hoc analysis was then performed to investigate whether the results were impacted by adjustment for further covariates, (i.e., a: alcohol intake, smoking, physical activity, and b: body mass index (BMI). P values were 2-sided. Statistical significance was set at < 0.05. All analyses were performed using the R Software for Statistical Computing, version 3.6.1 [43].
Results
Characteristics of the present cohort at the baseline UK Biobank visit
The mean age of the 104,895 participants at the baseline visit was 57.1 (SD 8.0) years. The majority of the cohort was female (54%) (Table 2). Almost half of the cohort had a higher education status (49.3%). Approximately one-fifth of the participants were classified, respectively, in the lowest or the highest SES quintile (least deprived 21.1%; most deprived 18.2%). In the VNR-test, the average number of correct responses was 6.1 (SD 2.1). The average diet score was 3.9 (SD 1.4). The majority of participants reported that their consumption of each of the individual diet score components was in line with the cut-offs predefined by Lourida et al. [19] (fruit: 53%; vegetables: 87%; fish: 54%; processed meat: 68%; unprocessed meat: 51%; refined grain: 69%). Nonetheless, only 9% of the participants reported the consumption of 3 servings of whole grain per day. Overall, the lifestyle of the participants was favorable, as almost 91% of the participants reported that they were current non-smokers, and 84% reported that they engaged in regular physical activity. Table 2 provides an overview of the baseline characteristics of the cohort. The PGS showed an approximately normal distribution (data not shown).
Association between fluid intelligence and diet score
In the unadjusted model, the diet score was positively associated with performance in the VNR-test (P < 0.0001). However, the magnitude of the effect was small (Cohen’s f2 = 0.001), since for participants with a high diet score, the estimated average difference in the number of correct responses was only 0.25 (95% CI 0.19–0.32) compared to participants with a low diet score. The association did not remain after adjustment for further confounders. After adjustment for age, sex, educational status, and the Townsend deprivation index, participants with an intermediate or a high diet score did not achieve more correct responses at baseline (P = 0.601) than participants with a low diet score (Table 3 and S1). In the adjusted model, no association was found between the continuous diet score and the VNR-test (beta = − 0.001; 95% CI − 0.01 − 0.01; Cohen’s f2 < 0.001, P = 0.780). Interestingly, however, slightly different effects were observed for the individual components of the diet score. In the basic model, that a higher intake of either fruits, vegetable, fish, or processed meat was associated with lower cognitive performance, while a higher intake of unprocessed meat, whole grain, or refined grain was associated with a lower cognitive performance. Whereas fruit intake was no longer associated with cognitive performance after adjustment (beta = − 0.01, CI − 0.03 − 0.02, P = 0.64), the associations for vegetables, fish, processed meat, unprocessed meat, whole grain, and refined grain remained (beta = − 0.04, CI − 0.07 to − 0.003, P = 0.03; beta = − 0.09, CI − 0.11 to − 0.06 P < 0.0001; beta = − 0.11, CI − 0.13 to − 0.08, P < 0.0001; beta = 0.02, CI 0.0001–0.57, P = 0.04; beta = 0.15, CI 0.11–0.19, P < 0.0001; and beta = 0.14, CI 0.11–0.16, P < 0.0001, respectively). Results of the analysis of the individual components of the diet score are provided in the Supplement (Table S2).
Association between fluid intelligence and the PGS
A positive association was found between the VNR-test at baseline and the PGS (Cohen’s f2 = 0.262). A similar increase in the number of correct responses was observed across all PGS categories (P < 0.0001). After adjustment for age, sex, educational status, the Townsend deprivation index, and the first 20 PCs, the estimated difference in the number of correct responses was 1.30 (95% CI 1.27–1.32) for participants with an intermediate PGS compared to participants with a low PGS. Similarly, on average, participants with a high PGS had three more correct responses on the baseline VNR-test (2.88; 95% CI 2.84–2.91) than participants with a low PGS (Table 3). Notably, these findings replicate those of a previous analysis conducted using data from the UK Biobank [26]. There was no evidence that the individual genetic disposition modifies the association between the diet score and performance in the VNR-test in the fully adjusted model (P-interaction = 0.051). No association was found between the diet score and performance in the VNR-test in any of their PGS strata (Table S4).
Fluid intelligence in relation to the diet score and genetic disposition
A model including both the diet score and the PGS was analyzed. The findings were comparable to those obtained in the analysis of fluid intelligence and either the diet score or the PGS alone. Taking the low PGS and low diet score categories as the reference participants, more correct responses in the VNR-test were observed among participants classified in the intermediate (around 1.3 more correct responses) and high (around 2.9 more correct responses) PGS strata, but no difference in VNR-test score was observed between the strata of the diet score. The number of correct responses in the VNR-test was comparable across the three diet score groups (low: 0–0.1, intermediate 1.34–1.37, and high 2.87–2.99) (Fig. 2).
Association between the VNR-test diet and the PGS in 104,895 participants from the UK Biobank. Coefficient estimates and P values were obtained from linear regression models adjusted for age, sex, education, the Townsend deprivation index, and PC 1–20. PC principal component, PGS polygenic risk score, VNR-test verbal-number-reasoning test
Change in fluid intelligence over time
For 9482 of the 104,895 participants, longitudinal data on fluid intelligence were available. These participants had attended follow-up visits at an average of 3.0 years (SD 0.3, first follow-up; n = 3,297) and 7.3 years (SD 1.3, second follow-up; n = 7,349) after the baseline UK Biobank visit. For the VNR-test, the mean change in the number of correct responses was 0.19 (baseline to first follow-up) and 0.03 (baseline to second follow-up). Thus, participants achieved slightly more correct responses when the test was completed a second or third time. However, change in VNR-test performance was similar in participants with a high diet score compared to those with a low diet score (P = 0.4227) (Table S3). Moreover, the inter-PGS group differences in VNR-test results remained constant over time (PInteraction = 0.2016).
Post hoc analysis
After adjustment for additional confounders (i.e., a alcohol intake, smoking, physical activity, and b: BMI) the results remained virtually unchanged (Table S4).
Discussion
The present analysis found no evidence that a diet score was associated with fluid intelligence. Furthermore, no evidence was found for the influence on the relationship between diet score and fluid intelligence of a PGS for general cognitive function [26]. Similar findings were observed in the investigation of the association with change in fluid intelligence over on average 6 year (range 2.1–9.7) period of follow-up.
The present results contrast with those of several previous observational studies involving both short and long follow-up periods [7, 9, 13, 44, 45], which reported modest associations between dietary patterns and cognitive health. A study from France found that adherence to a Mediterranean-type dietary pattern was associated with a less pronounced decline in performance in the Mini- Mental State examination (MMSE) [7]. Similarly, a study from Sweden found that a Western diet was associated with greater cognitive decline, while a prudent diet was associated with less cognitive decline [44]. In the same cohort, the Nordic diet was associated with a superior preservation of cognitive function [9]. Furthermore, a study in the US showed that the MIND-Diet was significantly associated with a slower decline in cognitive abilities [13]. Similarly, results from the Singapore Chinese Health Study showed that adherence to a “healthy dietary pattern” in midlife was associated with a lower risk for cognitive impairment in later life, as measured using a “Singapore-modified” MMSE [45]. In contrast, the present findings are consistent with the results of two previous long-term follow-up studies. In the Atherosclerosis Risk in Communities study, a 20-year change in global cognitive function was assessed using three cognitive tests at three time points across the follow-up period. The authors found no association between a Western and a prudent dietary pattern in midlife [46]. Similar findings were obtained in the Rancho Bernardo Study: whereas the MED diet was associated with superior global cognitive function at baseline, no association with cognitive decline over time was found for the alternate MED, the AHEI-2010, or a dietary pattern derived from factor analysis [47]. With the exception of one report [45], these previous studies investigated older individuals, whose average baseline ages range between ≥ 60 [44] and 81 years [13]. In contrast, the present study investigated younger participants (mean baseline age 57.1). Given that cognitive functioning declines with age, this may explain cross-study inconsistencies. Indeed, even in the UK Biobank, research has shown a significant correlation between performance in all of the administered cognitive tests and age. Older individuals in the UK Biobank showed a poorer performance in all but one test (absolute Pearson correlations for the respective test and age ranged from 0.16 to 0.60, P ≤ 0.040) [48]. Moreover, a previous study found a weak but significant negative association between age and fluid intelligence across three time points in UK Biobank participants [21]. Cornelis et al. showed that together with other cognitive functions, baseline fluid intelligence was lower in participants aged 65 + years compared to participants aged < 45 years. However, the authors concluded that declines in cognitive abilities below the age of 65 years are small [34]. Further potential explanations for the observed cross-study inconsistencies may include differences in terms of age and sex distributions, duration of follow-up, the investigated dietary patterns, and the dietary instruments used to obtain the dietary data. A further potential explanation for the observed cross-study inconsistencies is selection bias [49]. Previous authors have proposed the existence in the UK Biobank cohort of a “healthy volunteer” selection bias, since the participants were less likely to be obese, to smoke, and to drink alcohol on a daily basis, and had fewer self-reported health conditions compared with individuals from the general population. Research has found that on average, UK Biobank participants are more health-conscious than individuals from the general population [49]. This may explain the fact that a fairly high proportion of participants were current non-smokers (91%), engaged in regular physical activity (84%), or reported only a moderate level of alcohol consumption (67%) (Table 2). Furthermore, the present analysis included only around 20% (n = 104,895) of the 502,536 UK Biobank participants, and only 9% (n = 9482) of these 104,895 participants could be included in the longitudinal analyses due to limited data availability. The largest missing data rate was found for the present outcome variable (i.e., the VNR-test score), since this test was only used at 10 of the recruitment sites [34]. Previous authors have also suggested that measurement errors—which would contribute to type 2 errors—may be of concern for the cognitive tests conducted in the UK Biobank, and have pointed out that the issue of whether measurement error was random or varied according to age or time of recruitment remains unclear[34].
Two specific aspects of the present analyses warrant further discussion: the strongly reduced (and no longer significant) association for the diet score after adjustment for confounding factors; and the associations between the VNR-score and the individual dietary components. With respect to the latter, a recently published study using the UK Biobank resource observed similar associations between individual dietary components and a more comprehensive cognitive outcome than that used in the present analysis [11]. Here, Hepsomali and Groeger reported an inverse association between vegetable intake and a principal component analysis (PCA) derived score for general cognitive ability [11]. In general, previous research has established that both educational status [50] and SES [51] are associated with cognitive function. Therefore, an alteration in the effect of the diet score was expected and confirmed in the fully adjusted model in our study (Table S1, Table 3). Interestingly, slightly different attenuations after adjustments were observed for the individual components of the diet score. This could indicate the existence of differences in the association with fluid intelligence between the separate diet components, and in their interplay with the investigated covariates. Yet, in the present cohort, descriptive analyses of the covariates revealed a balanced distribution for educational status, SES, and sex for the individual dietary components, and in the regression models, the estimated effects of the covariates were almost identical (data not shown). Thus, these covariates may not explain the differences in the associations of the individual food groups that were observed in the multivariate models.
The present finding that individual genetic disposition did not influence the association between the diet score and the VNR-test is consistent with the recent study conducted by Lourida et al. [19] which found that a favorable lifestyle was associated with a lower risk of dementia independent of the genetic risk [19]. However, the authors did not report whether the diet score—which was included as one component of the lifestyle score—was itself associated with risk for incident dementia. In contrast, the population-based Rotterdam cohort study found that a healthy diet score, as based on adherence to the 2015 Dutch dietary guidelines, associated with a lower risk for dementia in participants with a low and intermediate genetic risk but not in participants with a high genetic risk [23]. Previous studies have generated inconsistent results concerning the role of genetic disposition for Alzheimer disease, i.e., the APOE ε4 genotype. In one study, the association between velocity of cognitive decline and different nutrient patterns varied depending on the APOE ε4 genotype [52]. Another study found that APOE ε4 carriers, but not APOE ε4 non-carriers, showed slower rates of decline in global cognition and in multiple cognitive domains in relation to n-3 fatty acid and seafood consumption [53]. In contrast, no effect of the APOE ε4 genotypes was found in two other studies [54, 55]. Notably, the APOE ε4 genotype was included in the PGS used, because it has been associated with general cognitive function in the most recent GWAS [26].
One potential explanation for the observed cross-study differences is the heterogeneity of the applied tests of cognitive function. Results from a validation study of 160 participants showed that among other measures of cognition used in UK Biobank, performance in the fluid intelligence test correlated well (Pearson correlation r > 0.55) with a general measure of cognitive ability that was based on a battery of standard neuropsychological tests. The authors suggest that the UK Biobank tests, including the fluid intelligence test, load strongly on general cognitive ability [48]. Hence, the VNR-test administrated in the UK Biobank seems to be a reliable and valid measure of cognitive performance. Although all cognitive tests correlate positively, whereby an individual who performs well on one test will tend to perform well on others [24, 56], they do not measure the same ability. As discussed elsewhere, inter-individual differences in cognitive test performance may be attributable to differences that are specific to: (1) a given test, (2) a particular cognitive domain, and/or (3) the existence of a construct known as general cognitive ability [24, 57]. Interestingly, of the four neuropsychological tests used to assess cognitive performance in the French Three-City study, i.e., MMSE, Isaacs Set Test, Benton Visual Retention Test, and Free and Cued Selective Reminding Test, stricter adherence to a MED diet was associated with slower cognitive decline when measured using the MMSE, whereas no consistent association was found for any of the three remaining tests [7].
The present study had several limitations. First, the healthy-volunteer selection bias may partly explain why, in contrast to previous studies, no association between the diet score and performance in the VNR-test was found [49]. Second, the analyses did not consider the issue of total energy intake. Energy adjustment is a common method in nutritional epidemiology [58]. Thus, investigations of diet–disease relationships usually consider total energy intake as a confounding variable. In the present study, this was not possible, since the investigated dietary data were derived from a 29-item questionnaire on main food groups, which did not encompass total dietary intake. However, after adjustment for BMI, which may serve as a proxy of energy expenditure [11, 59], the results remained virtually unchanged. Third, the cognitive outcome was the result of the VNR-test, which measures fluid intelligence only [37]. Moreover, the VNR-test does not capture pathophysiological disorders, such as mild cognitive impairment or dementia, and does not reflect the impairments of cognitive performance that are characteristic of these conditions. Thus since fluid intelligence reflects only one cognitive domain, the results relating to the present diet score may not be transferable to other cognitive domains. Fourth, the cohort comprised middle-aged participants of Caucasian ancestry only. This demographic profile may limit the generalizability of the findings. However, the homogeneity of the study sample, and the resultant internal validity, may have limited potential confounding effects, such as SES, educational status, or ancestry. Finally, although the analyses controlled for a range of confounding factors, residual confounders may exist.
Since results from previous cohort studies of the association between diet/dietary pattern and cognitive function might have been inconclusive due to limited sample sizes, variation in age and gender distributions, and/or cross-study differences in phenotypic and exposure measurements, the present study also had several strengths. First, to our knowledge, it represents the largest single investigation of dietary patterns in relation to fluid intelligence to date. Second, all participants had undergone a uniform assessment of both their dietary intake and cognitive phenotype. Third, the comprehensive nature of the UK Biobank data enabled lifestyle factors such as educational status and SES to be taken into account. Fourth, the analyses considered genetic disposition, which is an advantage when investigating a multifactorial phenotype such as cognitive function.
In conclusion, no evidence was found for an association between fluid intelligence and a dietary score that considered fruit, vegetables, fish, processed meat, unprocessed meat, whole grain, and refined grain either at baseline or longitudinally. Results did not materially change after consideration of individual genetic disposition for cognitive function. Further studies, in particular long-term follow-up investigations, are warranted. Future research questions could be extended to more comprehensive or exploratory derived dietary patterns as well as the consideration of different cognitive domains.
Data availability
Data described in the manuscript will not be made available, because we do not have the permission to share these data, but it can be applied at the UK Biobank resource at https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access.
Code availability
The code book and analytic code will be made available upon request pending application and approval.
References
Dauncey MJ (2014) Nutrition, the brain and cognitive decline: insights from epigenetics. Eur J Clin Nutr 68(11):1179–1185. https://doi.org/10.1038/ejcn.2014.173
Gomez-Pinilla F, Tyagi E (2013) Diet and cognition: interplay between cell metabolism and neuronal plasticity. Curr Opin Clin Nutr Metab Care 16(6):726–733. https://doi.org/10.1097/MCO.0b013e328365aae3
Dauncey MJ (2009) New insights into nutrition and cognitive neuroscience. Proc Nutr Soc 68(4):408–415. https://doi.org/10.1017/S0029665109990188
Martinez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvado J, San Julian B, Sanchez-Tainta A, Ros E, Valls-Pedret C, Martinez-Gonzalez MA (2013) Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry 84(12):1318–1325. https://doi.org/10.1136/jnnp-2012-304792
Marchand NE, Jensen MK (2018) The role of dietary and lifestyle factors in maintaining cognitive health. Am J Lifestyle Med 12(4):268–285. https://doi.org/10.1177/1559827617701066
Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ (2013) Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology 24(4):479–489. https://doi.org/10.1097/EDE.0b013e3182944410
Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P (2009) Adherence to a mediterranean diet, cognitive decline, and risk of dementia. JAMA 302(6):638–648. https://doi.org/10.1001/jama.2009.1146
Radd-Vagenas S, Duffy SL, Naismith SL, Brew BJ, Flood VM, Fiatarone Singh MA (2018) Effect of the mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am J Clin Nutr 107(3):389–404. https://doi.org/10.1093/ajcn/nqx070
Shakersain B, Rizzuto D, Larsson SC, Faxen-Irving G, Fratiglioni L, Xu WL (2018) The nordic prudent diet reduces risk of cognitive decline in the swedish older adults: a population-based cohort study. Nutrients. https://doi.org/10.3390/nu10020229
Mannikko R, Komulainen P, Schwab U, Heikkila HM, Savonen K, Hassinen M, Hanninen T, Kivipelto M, Rauramaa R (2015) The nordic diet and cognition–The DR’s EXTRA Study. Br J Nutr 114(2):231–239. https://doi.org/10.1017/S0007114515001890
Hepsomali P, Groeger JA (2021) Diet and general cognitive ability in the UK Biobank dataset. Sci Rep 11(1):11786. https://doi.org/10.1038/s41598-021-91259-3
Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT (2015) MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 11(9):1007–1014. https://doi.org/10.1016/j.jalz.2014.11.009
Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT (2015) MIND diet slows cognitive decline with aging. Alzheimers Dement 11(9):1015–1022. https://doi.org/10.1016/j.jalz.2015.04.011
Mozaffarian D (2016) Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 133(2):187–225. https://doi.org/10.1161/CIRCULATIONAHA.115.018585
Stewart RAH, Held C, Krug-Gourley S, Waterworth D, Stebbins A, Chiswell K, Hagstrom E, Armstrong PW, Wallentin L, White H (2019) Cardiovascular and lifestyle risk factors and cognitive function in patients with stable coronary heart disease. J Am Heart Assoc 8(7):e010641. https://doi.org/10.1161/JAHA.118.010641
Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C (2011) Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77(5):461–468. https://doi.org/10.1212/WNL.0b013e318227b227
Plassman BL, Williams JW Jr, Burke JR, Holsinger T, Benjamin S (2010) Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med 153(3):182–193. https://doi.org/10.7326/0003-4819-153-3-201008030-00258
Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S (2014) Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 129(15):1560–1567. https://doi.org/10.1161/CIRCULATIONAHA.113.004798
Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hypponen E, Kuzma E, Llewellyn DJ (2019) Association of lifestyle and genetic risk with incidence of dementia. JAMA. https://doi.org/10.1001/jama.2019.9879
Gray JR, Chabris CF, Braver TS (2003) Neural mechanisms of general fluid intelligence. Nat Neurosci 6(3):316–322. https://doi.org/10.1038/nn1014
Kievit RA, Fuhrmann D, Borgeest GS, Simpson-Kent IL, Henson RNA (2018) The neural determinants of age-related changes in fluid intelligence: a pre-registered, longitudinal analysis in UK Biobank. Wellcome Open Res 3:38. https://doi.org/10.12688/wellcomeopenres.14241.2
Tucker-Drob EM (2011) Neurocognitive functions and everyday functions change together in old age. Neuropsychology 25(3):368–377. https://doi.org/10.1037/a0022348
Licher S, Ahmad S, Karamujic-Comic H, Voortman T, Leening MJG, Ikram MA, Ikram MK (2019) Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med 25(9):1364–1369. https://doi.org/10.1038/s41591-019-0547-7
Davies G, Marioni RE, Liewald DC, Hill WD, Hagenaars SP, Harris SE, Ritchie SJ, Luciano M, Fawns-Ritchie C, Lyall D, Cullen B, Cox SR, Hayward C, Porteous DJ, Evans J, McIntosh AM, Gallacher J, Craddock N, Pell JP, Smith DJ, Gale CR, Deary IJ (2016) Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N=112 151). Mol Psychiatry 21(6):758–767. https://doi.org/10.1038/mp.2016.45
Trampush JW, Yang ML, Yu J, Knowles E, Davies G, Liewald DC, Starr JM, Djurovic S, Melle I, Sundet K, Christoforou A, Reinvang I, DeRosse P, Lundervold AJ, Steen VM, Espeseth T, Raikkonen K, Widen E, Palotie A, Eriksson JG, Giegling I, Konte B, Roussos P, Giakoumaki S, Burdick KE, Payton A, Ollier W, Horan M, Chiba-Falek O, Attix DK, Need AC, Cirulli ET, Voineskos AN, Stefanis NC, Avramopoulos D, Hatzimanolis A, Arking DE, Smyrnis N, Bilder RM, Freimer NA, Cannon TD, London E, Poldrack RA, Sabb FW, Congdon E, Conley ED, Scult MA, Dickinson D, Straub RE, Donohoe G, Morris D, Corvin A, Gill M, Hariri AR, Weinberger DR, Pendleton N, Bitsios P, Rujescu D, Lahti J, Le Hellard S, Keller MC, Andreassen OA, Deary IJ, Glahn DC, Malhotra AK, Lencz T (2017) GWAS meta-analysis reveals novel loci and genetic correlates for general cognitive function: a report from the COGENT consortium. Mol Psychiatry 22(3):336–345. https://doi.org/10.1038/mp.2016.244
Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, Hagenaars SP, Ritchie SJ, Marioni RE, Fawns-Ritchie C, Liewald DCM, Okely JA, Ahola-Olli AV, Barnes CLK, Bertram L, Bis JC, Burdick KE, Christoforou A, DeRosse P, Djurovic S, Espeseth T, Giakoumaki S, Giddaluru S, Gustavson DE, Hayward C, Hofer E, Ikram MA, Karlsson R, Knowles E, Lahti J, Leber M, Li S, Mather KA, Melle I, Morris D, Oldmeadow C, Palviainen T, Payton A, Pazoki R, Petrovic K, Reynolds CA, Sargurupremraj M, Scholz M, Smith JA, Smith AV, Terzikhan N, Thalamuthu A, Trompet S, van der Lee SJ, Ware EB, Windham BG, Wright MJ, Yang J, Yu J, Ames D, Amin N, Amouyel P, Andreassen OA, Armstrong NJ, Assareh AA, Attia JR, Attix D, Avramopoulos D, Bennett DA, Bohmer AC, Boyle PA, Brodaty H, Campbell H, Cannon TD, Cirulli ET, Congdon E, Conley ED, Corley J, Cox SR, Dale AM, Dehghan A, Dick D, Dickinson D, Eriksson JG, Evangelou E, Faul JD, Ford I, Freimer NA, Gao H, Giegling I, Gillespie NA, Gordon SD, Gottesman RF, Griswold ME, Gudnason V, Harris TB, Hartmann AM, Hatzimanolis A, Heiss G, Holliday EG, Joshi PK, Kahonen M, Kardia SLR, Karlsson I, Kleineidam L, Knopman DS, Kochan NA, Konte B, Kwok JB, Le Hellard S, Lee T, Lehtimaki T, Li SC, Lill CM, Liu T, Koini M, London E, Longstreth WT Jr, Lopez OL, Loukola A, Luck T, Lundervold AJ, Lundquist A, Lyytikainen LP, Martin NG, Montgomery GW, Murray AD, Need AC, Noordam R, Nyberg L, Ollier W, Papenberg G, Pattie A, Polasek O, Poldrack RA, Psaty BM, Reppermund S, Riedel-Heller SG, Rose RJ, Rotter JI, Roussos P, Rovio SP, Saba Y, Sabb FW, Sachdev PS, Satizabal CL, Schmid M, Scott RJ, Scult MA, Simino J, Slagboom PE, Smyrnis N, Soumare A, Stefanis NC, Stott DJ, Straub RE, Sundet K, Taylor AM, Taylor KD, Tzoulaki I, Tzourio C, Uitterlinden A, Vitart V, Voineskos AN, Kaprio J, Wagner M, Wagner H, Weinhold L, Wen KH, Widen E, Yang Q, Zhao W, Adams HHH, Arking DE, Bilder RM, Bitsios P, Boerwinkle E, Chiba-Falek O, Corvin A, De Jager PL, Debette S, Donohoe G, Elliott P, Fitzpatrick AL, Gill M, Glahn DC, Hagg S, Hansell NK, Hariri AR, Ikram MK, Jukema JW, Vuoksimaa E, Keller MC, Kremen WS, Launer L, Lindenberger U, Palotie A, Pedersen NL, Pendleton N, Porteous DJ, Raikkonen K, Raitakari OT, Ramirez A, Reinvang I, Rudan I, Dan R, Schmidt R, Schmidt H, Schofield PW, Schofield PR, Starr JM, Steen VM, Trollor JN, Turner ST, Van Duijn CM, Villringer A, Weinberger DR, Weir DR, Wilson JF, Malhotra A, McIntosh AM, Gale CR, Seshadri S, Mosley TH Jr, Bressler J, Lencz T, Deary IJ (2018) Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun 9(1):2098. https://doi.org/10.1038/s41467-018-04362-x
Sniekers S, Stringer S, Watanabe K, Jansen PR, Coleman JRI, Krapohl E, Taskesen E, Hammerschlag AR, Okbay A, Zabaneh D, Amin N, Breen G, Cesarini D, Chabris CF, Iacono WG, Ikram MA, Johannesson M, Koellinger P, Lee JJ, Magnusson PKE, McGue M, Miller MB, Ollier WER, Payton A, Pendleton N, Plomin R, Rietveld CA, Tiemeier H, van Duijn CM, Posthuma D (2017) Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat Genet 49(7):1107–1112. https://doi.org/10.1038/ng.3869
Maher BS (2015) Polygenic scores in epidemiology: risk prediction, etiology, and clinical utility. Curr Epidemiol Rep 2(4):239–244. https://doi.org/10.1007/s40471-015-0055-3
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R (2015) UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12(3):e1001779. https://doi.org/10.1371/journal.pmed.1001779
Ollier W, Sprosen T, Peakman T (2005) UK Biobank: from concept to reality. Pharmacogenomics 6(6):639–646. https://doi.org/10.2217/14622416.6.6.639
UKBiobank The Ethics and Governance Council. https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics. Accessed 18 Sep 2021
UKBiobank Location of the UK Biobank assessment centers throughout the United Kingdom. https://biobank.ndph.ox.ac.uk/showcase/exinfo.cgi?src=UKB_centres_map. Accessed 18 Sep 2021
UK Biobank Access Management System (AMS) User Guide: Getting Started
Cornelis MC, Wang Y, Holland T, Agarwal P, Weintraub S, Morris MC (2019) Age and cognitive decline in the UK Biobank. PLoS ONE 14(3):e0213948. https://doi.org/10.1371/journal.pone.0213948
Cullen B, Nicholl BI, Mackay DF, Martin D, Ul-Haq Z, McIntosh A, Gallacher J, Deary IJ, Pell JP, Evans JJ, Smith DJ (2015) Cognitive function and lifetime features of depression and bipolar disorder in a large population sample: cross-sectional study of 143,828 UK Biobank participants. Eur Psychiatry 30(8):950–958. https://doi.org/10.1016/j.eurpsy.2015.08.006
Category 118 Fluid intelligence/reasoning, Cognitive function online follow-up. https://biobank.ctsu.ox.ac.uk/crystal/label.cgi?id=116. Accessed 20 May 2022
UKBiobank Fluid-intelligence measurement using ACE https://biobank.ndph.ox.ac.uk/showcase/refer.cgi?id=100231. Accessed 18 Sep 2021
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, Cortes A, Welsh S, Young A, Effingham M, McVean G, Leslie S, Allen N, Donnelly P, Marchini J (2018) The UK Biobank resource with deep phenotyping and genomic data. Nature 562(7726):203–209. https://doi.org/10.1038/s41586-018-0579-z
UKBiobank Data showcase Data-Field 20016—Fluid intelligence score. https://biobank.ndph.ox.ac.uk/showcase/field.cgi?id=20016. Accessed 18 Sep 2021
The Haplotype Reference Consortium
Townsend P (1987) Deprivation. J Soc Policy 16(2):125–146
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191. https://doi.org/10.3758/bf03193146
Team RC (2019) R: A language and environment for statistical computing. R Foundation for Statistical
Shakersain B, Santoni G, Larsson SC, Faxen-Irving G, Fastbom J, Fratiglioni L, Xu W (2016) Prudent diet may attenuate the adverse effects of western diet on cognitive decline. Alzheimers Dement 12(2):100–109. https://doi.org/10.1016/j.jalz.2015.08.002
Wu J, Song X, Chen GC, Neelakantan N, van Dam RM, Feng L, Yuan JM, Pan A, Koh WP (2019) Dietary pattern in midlife and cognitive impairment in late life: a prospective study in Chinese adults. Am J Clin Nutr 110(4):912–920. https://doi.org/10.1093/ajcn/nqz150
Dearborn-Tomazos JL, Wu A, Steffen LM, Anderson CAM, Hu EA, Knopman D, Mosley TH, Gottesman RF (2019) Association of dietary patterns in midlife and cognitive function in later life in US adults without dementia. JAMA Netw Open 2(12):e1916641. https://doi.org/10.1001/jamanetworkopen.2019.16641
Richard EL, Laughlin GA, Kritz-Silverstein D, Reas ET, Barrett-Connor E, McEvoy LK (2018) Dietary patterns and cognitive function among older community-dwelling adults. Nutrients. https://doi.org/10.3390/nu10081088
Fawns-Ritchie C, Deary IJ (2020) Reliability and validity of the UK Biobank cognitive tests. PLoS ONE 15(4):e0231627. https://doi.org/10.1371/journal.pone.0231627
Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE (2017) Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 186(9):1026–1034. https://doi.org/10.1093/aje/kwx246
Lovden M, Fratiglioni L, Glymour MM, Lindenberger U, Tucker-Drob EM (2020) Education and cognitive functioning across the life span. Psychol Sci Public Interest 21(1):6–41. https://doi.org/10.1177/1529100620920576
Hackman DA, Farah MJ, Meaney MJ (2010) Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci 11(9):651–659. https://doi.org/10.1038/nrn2897
Prinelli F, Fratiglioni L, Musicco M, Johansson I, Adorni F, Shakersain B, Rizzuto D, Xu W (2019) The impact of nutrient-based dietary patterns on cognitive decline in older adults. Clin Nutr 38(6):2813–2820. https://doi.org/10.1016/j.clnu.2018.12.012
van de Rest O, Wang Y, Barnes LL, Tangney C, Bennett DA, Morris MC (2016) APOE epsilon4 and the associations of seafood and long-chain omega-3 fatty acids with cognitive decline. Neurology 86(22):2063–2070. https://doi.org/10.1212/WNL.0000000000002719
Samieri C, Morris MC, Bennett DA, Berr C, Amouyel P, Dartigues JF, Tzourio C, Chasman DI, Grodstein F (2018) Fish intake, genetic predisposition to alzheimer disease, and decline in global cognition and memory in 5 cohorts of older persons. Am J Epidemiol 187(5):933–940. https://doi.org/10.1093/aje/kwx330
Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martinez-Gonzalez MA, Martinez-Lapiscina EH, Fito M, Perez-Heras A, Salas-Salvado J, Estruch R, Ros E (2015) Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med 175(7):1094–1103. https://doi.org/10.1001/jamainternmed.2015.1668
Carroll JB (1993) Human cognitive abilities: a survey of factor-analytic studies. Cambridge University Press, Cambridge
Deary IJ (2013) Intelligence. Curr Biol 23(16):R673-676. https://doi.org/10.1016/j.cub.2013.07.021
Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65(4 Suppl):1220S-1228S. https://doi.org/10.1093/ajcn/65.4.1220S (discussion 1229S-1231S)
Jakes RW, Day NE, Luben R, Welch A, Bingham S, Mitchell J, Hennings S, Rennie K, Wareham NJ (2004) Adjusting for energy intake–what measure to use in nutritional epidemiological studies? Int J Epidemiol 33(6):1382–1386. https://doi.org/10.1093/ije/dyh181
Acknowledgements
This research has been conducted using the UK Biobank Resource. Access to the UK Biobank data was granted under Application Number 31615-Genetic factors as a biological link between food intake and cognition.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by Diet–Body–Brain (DietBB). The DietBB Competence Cluster in Nutrition Research is funded by the German Federal Ministry of Education and Research (FKZ: 01EA1410A).
Author information
Authors and Affiliations
Contributions
Conception and the design of the study: CAS and UN; methodology and data analysis: CAS and LW; interpretation: CAS, LW, MS, UN, and MN; manuscript preparation: CAS; all authors: critically revised the manuscript and read and approved the final manuscript; primary responsibility for final content: UN; funding acquisition: MS, UN, and MN.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interests to declare in relation to this study.
Ethical approval
The ethical approval process for the UK Biobank study is described elsewhere [31]. In Brief, ethical approval for the study had been obtained by the National Information Governance Board for Health and Social Care and the National Health Service North West Multicenter Research Ethics Committee.
Informed consent
All participants provided informed consent through electronic signature at baseline [31].
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schulz, CA., Weinhold, L., Schmid, M. et al. Analysis of associations between dietary patterns, genetic disposition, and cognitive function in data from UK Biobank. Eur J Nutr 62, 511–521 (2023). https://doi.org/10.1007/s00394-022-02976-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02976-y