Abstract
Purpose
Milk-derived free fatty acids (FFAs) may act as both biomarkers of intake and metabolic effect. In this study we explored associations between different types of dairy consumption, a selection of milk-derived free fatty acids, and cardiometabolic disease (CMD) risk factors.
Methods
Sixty-seven FFAs were quantified in the plasma of 131 free-living Dutch adults (median 60 years) using gas chromatography-flame ionization detector. Intakes of different dairy foods and groups were assessed using a food frequency questionnaire. Twelve different CMD risk factors were analyzed. Multiple linear regressions were used to evaluate the associations under study.
Results
Based on the fully adjusted models, 5 long-chain unsaturated FFAs (C18:1 t13 + c6 + c7 + u, C18:2 c9t11 + u, C20:1 c11, C20:3 c8c11c14, and C20:4 c5c8c11c14), 2 medium-chain saturated FFAs (C15, C15 iso), and a trans FFA (C16:1 t9) were positively associated with at least one variable of dairy intake, as well as plasma total and LDL cholesterol, blood pressure, and SCORE (p ≤ 0.05). A long-chain PUFA associated with high-fat fermented dairy intake (C18:2 t9t12), was negatively associated with serum triglyceride levels, and a long-chain saturated FFA associated with cheese intake (C18:1 u1) was negatively associated with plasma LDL cholesterol and serum triglyceride levels. No clear associations were observed between dairy intake and CMD risk factors.
Conclusion
Milk-derived FFAs could act as sensitive biomarkers for dairy intake and metabolism, allowing the association between dairy and CMD risk to be more precisely evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiometabolic diseases (CMDs), encompassing cardiovascular disease and type II diabetes, represent one of the largest health and socioeconomic burdens to modern society. In Europe, cardiovascular disease morbidity affects more than 85 million people, leading to ~ 4 million annual deaths [1]. Proper nutrition is considered the primary lifestyle approach for preventing and managing CMD risk. Diets abundant in fruits, vegetables, whole grains, nuts, and legumes contribute to lowering CMD risk, while consumption of processed meats, refined grains, and sugar-sweetened beverages are considered detrimental [2]. Particular nutrients in foods have also been adversely associated with CMD risk, including sugar, sodium, trans (unsaturated) fat, and saturated fat [2].
Dairy products have had a contested role in the dietary management of CMDs due to suggested beneficial as well as adverse health effects. While dairy foods are rich in macro- and micronutrients considered important for growth and development, they can also have a high saturated fat content (contributing 25–30% of all saturated fat intake in the European diet) [3]. Earlier studies conducted in the 1960–70 s have reported that saturated fat adversely affects low-density lipoprotein cholesterol (LDL-C) levels in blood, which in turn increases CMD pathogenesis and progression [3, 4]. In view of this, many subsequent observational studies have compared high- and low-fat dairy intake on CMD risk, but the findings have been equivocal. A meta-analysis of prospective cohort studies revealed that total and low-fat dairy intake (but not high-fat dairy intake) was associated with a lower risk of hypertension [5], while several studies have revealed the merits of consuming full-fat dairy products on reducing central adiposity risk [6, 7] and increasing serum high-density lipoprotein cholesterol (HDL-C) [8]. In a recent systematic review of randomized controlled trials, Duarte et al. [9] reported that the consumption of dairy products (as a source of saturated fat) may improve some CMD risk factors compared with consumption of other animal sources of saturated fat. This finding lends support to the importance of considering the whole dairy matrix on disease outcomes. An increasing body of evidence also supports the differential role of distinct dairy foods with unique nutritional and/or microbial profiles on CMD risk [10]. For instance, consumption of yoghurt, a fermented dairy food, has been consistently associated with a reduced risk of type II diabetes [11] and combined cardiovascular disease [12].
A limitation of the above studies (which could partly explain the equivocal findings) is the reliance on subjective, self-reported dietary assessment methods. Here, food intake biomarkers (FIBs) could act as a more objective and accurate strategy for estimating dairy intake, and can lead to more consistent findings for associations between dairy intake and CMD risk factors [13]. Several fatty acids have been proposed as FIBs for dairy fat intake, including pentadecanoic acid (C15), heptadecanoic acid (C17), and trans-palmitoleic acid (C16:1 t9) [14,15,16,17,18,19,20], but these FIBs have not been thoroughly validated, and/or cannot discriminate the intake of specific dairy foods. Additionally, milk fat is a highly complex mixture of several thousand species of lipids, including ~ 400 fatty acids, which have distinct physiological importance and nutritive potential [21, 22]. Longer chain saturated fatty acids (e.g., C14, C16) as well as certain trans fatty acids are known to positively associate with total and LDL-C [23] and type II diabetes risk [24], while saturated fatty acids with shorter chain length (e.g., C6-C10), monounsaturated, and polyunsaturated fatty acids (MUFA and PUFA) are generally regarded as cardio-protective [25,26,27]. Surprisingly little is known about the relationships between milk-derived fatty acids with dairy intake, or how they associate with CMD risk. Recently, Drouin-Cartier et al. [25] evaluated plasma metabolite profiles associated with total dairy intake and risk of type II diabetes, and found 38 metabolites associated with dairy intake and lower type II diabetes risk. However, such comprehensive studies are fairly scarce and there remains a need to consider the interplay between different dairy foods, lipids and metabolites, and CMD risk factors.
The objective of the current study is therefore to evaluate associations between dairy intake, milk-derived free fatty acids (FFAs), and CMD risk parameters in a free-living adult population. To achieve a comprehensive analysis, we targeted 67 FFAs with high abundance in milk and looked at their associations with different dairy intake groups (dairy fat, fermented and non-fermented dairy, and specific dairy foods), and a wide range of CMD risk factors and composite risk scores.
Methods
Study design and population
The Nutrition Questionnaires plus (NQplus) study is a prospective cohort study comprising 2048 Dutch adults (20–70 years) living in or around Wageningen, The Netherlands. NQplus was initiated as an ‘add-on’ study to the National Dietary Assessment Reference Database (NDARD) project, and full details of NQplus and NDARD have been published previously [28, 29]. Participants were recruited and enrolled in the study between June 2011 and February 2013. Extensive data were collected at baseline, including an assessment of habitual dietary intake by food frequency questionnaire (FFQ) and/or 24-h recall, background demographics, health, anthropometric, and lifestyle data. Fasting blood samples and 24-h urine samples were also collected. The study was approved by the ethical committee of Wageningen University & Research and conducted in agreement with the Declaration of Helsinki. Written informed consent was obtained from all participants prior to the start of the study.
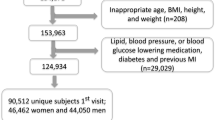
For the present study, we selected n = 131 NQplus participants for targeted FFA analysis from n = 228 who had a plasma sample collected within ± 14 days of completing an FFQ [30]. The selection of this smaller group was necessary to attain a number of samples that could be reasonably measured within time and economic restraints. To ensure a balance of participants across all dairy groups/foods assessed, ~ 20 participants were randomly selected from the low (Q1), mid (Q3), and high (Q5) quintiles of intake of each dairy group/food assessed.
Food frequency questionnaire and levels of dairy consumption
A full description of the semi-quantitative FFQ used to assess habitual dietary intake has been described in the study design papers for NQplus and NDARD [28, 29]. The semi-quantitative FFQ was self-administered and completed online, with ten frequency categories ranging from ‘never’ to ‘6–7 days per week’. Portion sizes were estimated using commonly used household measures. Total food or nutrient intakes (in g/day) were determined by multiplying consumption frequency by portion size and nutrient content as defined in the Dutch food composition tables [31]. Out of 216 total food items in the FFQ, 39 were identified as dairy products, which were further classified into dairy subgroups (Table S1). This FFQ has been previously validated for various dairy foods and food groups, including milk, yogurt, cheese, total fermented dairy (FD), and total non-fermented dairy (NFD) (against multiple 24-h recalls) [32], which are used in the current study for evaluation of the respective candidate FIBs. Total dairy, FD, and NFD groups were further stratified into high-fat groups, which included all full-fat dairy products, and low-fat groups, which included semi-skim and skim dairy products. Fat content (g/100 g) for all dairy products was determined based on the values reported in the Dutch Food Composition Table [31] and classifications of products as skim, semi-skim, and full-fat were based on the guidelines set by the Dutch Dairy Commodities Act (see Table S1).
Cardiometabolic disease risk parameters
A full description of the CMD risk parameters collected for NQplus has been described previously [29]. Height and weight were determined using a stadiometer (SECA, Germany, nearest 0.1 cm) and a digital weighing scale (SECA, nearest 0.1 kg), respectively. BMI was calculated by dividing weight (in kg) by height (in m2). Waist circumference was determined using a non-flexible measuring tape (SECA 201, nearest 0.5 cm); measurements were taken twice and averaged. Systolic and diastolic blood pressure (SBP and DBP) was measured using a digital blood pressure monitor (IntelliSense HEM-907, Omron Healthcare, USA); the first measurement was omitted and the second to up to the sixth measurement were averaged. Fasting plasma glucose, total, HDL-C, and serum triglycerides were analyzed using Dimension Vista 1500 automated analyser (Siemens, Erlangen, Germany) or Roche Modular P800 chemistry analyser (Roche Diagnostics, Indianapolis, USA). Plasma LDL-C was calculated with the Friedewald equation [33]. Blood hemoglobin A1c (HbA1c) was determined with HPLC (ADAMS A1c HA-8160 analyser, A. Menarini Diagnostics). Participants were characterized as having hypertension, suboptimal cholesterol, or type II diabetes based on the cut-offs and definitions described in relevant guidelines of the European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) [34,35,36], and having metabolic syndrome based on the harmonized guidelines of the International Diabetes Federation (IDF) et al. [37].
Two further composite risk scores were included in our analyses. Firstly, a continuous metabolic syndrome (MetS) score was constructed based on summed age- and sex-adjusted standardized residuals (z-scores) of all individual MetS parameters that incorporates plasma glucose, SBP, DBP, HDL-C, serum triglycerides, waist circumference as risk factors [38,39,40]. Since HDL-C is inversely associated with CMD risk, residuals for this parameter were multiplied by − 1 prior to summing. Secondly, the 10-year risk of fatal cardiovascular disease was evaluated using the European Systematic COronary Risk Evaluation (SCORE) low-risk country chart [41, 42], which incorporates sex, age, smoking status, SBP, and total cholesterol as risk factors. For the calculation of SCORE, a ‘smoker’ was considered to encompass current smokers and former smokers who quit > 35 years old, and ‘non-smoker’ as never smokers and former smokers who quit < 35 years old, as this age cut-off has been previously documented as a critical smoking cessation age to prolong life expectancy [43].
Covariates
Data on education level, smoking status, physical activity, and alcohol consumption were collected via standardized questionnaires [29]. Participants were classified as having ‘low’, ‘intermediate’, or ‘high’ education based on their highest levels of completed education (no education or primary/lower vocational education, lower secondary or intermediate vocational education, or higher secondary/vocational education or university). The criteria used to define ‘smoker’ and ‘non-smoker’ are outlined above. Information on the participants’ usual physical activity (min/week spent on sedentary, light, moderate and vigorous intensity activities) over the past 4 weeks was obtained using the validated Activity Questionnaire for Adults and Adolescents (AQuAA) [44]. Intake levels of alcohol and different foods were assessed by FFQ. Covariate selection was based on the current scientific literature and statistical testing (as described in the statistical analysis).
Measurement of free fatty acid concentrations in plasma
Ethylenediaminetetraacetic acid (EDTA) plasma collected for NQplus was stored in the biobank at − 80 °C. The targeted panel comprised 67 FFAs previously detected with the highest abundance in the milk fat of Swiss cows [45]. In addition, several FFA groups were determined based on the sums of individual FFAs (Table S2). Immediately prior to analysis, plasma samples were thawed on ice and were prepared for analysis by adding 15 µL of the internal standard (C13, 7 µg/15 µL) to 100 µL of plasma, followed by methylation of FFAs with MeOH/HCl at 25 °C for 45 min using methods described previously [46, 47]. A post-reaction treatment for neutralization was performed with 350 µL Na2CO3, and extraction was performed with 300 µL hexane. The FFA concentrations in plasma were determined using 0.5 µL injection of the hexane solution and analyzed using an Agilent 6890 high-resolution gas chromatograph equipped with a capillary column (100-m CP-Sil 88, Varian BV, Middleburg, Netherlands) and a flame ionization detector.
Statistical analysis
Participant characteristics are reported as mean (standard deviation) for normally distributed variables, or medians (interquartile range) for skewed variables. To permit comparability with a previous study where we assessed correlations between plasma C15 and C17 with various dairy groups [30], Spearman’s correlation coefficients (rs) were calculated as an initial step to assess the strength of the associations between dairy intakes (energy-adjusted g/day) and FFA concentrations (mg/L plasma); p-values are presented as raw and false discovery rate (FDR)-adjusted using the method of Benjamini and Hochberg [48]. Correlation coefficients of ≥ 0.50 were considered good, 0.20–0.49 as moderate, and < 0.20 as poor (39).
Multivariable adjusted linear regression and restricted cubic spline regression were used to evaluate the associations between self-reported dairy intakes, FFAs, and CMD risk factors. The assumption of linear relationships between exposure and outcome variables were tested using likelihood ratio tests of model deviance and Wald tests of spline coefficients. If tests were statistically significant, associations were visually inspected to confirm the presence of a true non-linear relationship and not due to artificial curves driven by outliers. All tests for non-linearity revealed that the dose–response association of dairy intake with FFAs and CMD risk parameters, and between FFAs and CMD risk parameters, could be considered linear. Thus, only linear regressions are presented in the results. Prior to association analyses, intakes of dairy foods were energy-adjusted using the commonly used residual method [49]. FFAs that were not detected in at least a third of participants were removed to select the most suitable candidate FIBs of dairy intake. CMD risk parameters acting as dependent variables that were not normally distributed (BMI, HbA1c, plasma glucose, serum triglycerides, and SCORE) were log transformed. Additionally, all variables were normalized by z-scores prior to analysis to allow comparability across associations. Analyses were performed unadjusted (Model 0), adjusted for age and sex (Model 1) + physical activity, smoking, and education level (Model 2) + dietary factors (Model 3). For associations with continuous MetS as a dependent variable, which already takes into account age and sex, analyses were performed unadjusted (Model 0) and fully adjusted for smoking, physical activity, education, and dietary factors (Model 3). For associations with SCORE as a dependent variable, which already takes into account age, sex, and smoking status, analyses were performed unadjusted (Model 0) and fully adjusted for physical activity, education, and dietary factors (Model 3). Dietary factors included in the fully adjusted models were dependent on the association studied, and included fish (dairy intake and FFAs), or alcohol, vegetables, fruits, and meat (dairy intake and CMD risk factors, FFAs and CMD risk factors). No other foods or food groups (listed in Table 1) were found to be strongly correlated with dairy intake, FFAs, or with CMD risk factors, and were thus not included in the models.
Further, we intended to examine potential mediation of the association between dairy intake and CMD risk factors by milk-derived FFAs by independently adding the FFAs to fully adjusted regression models. However, since there were no clear associations between dairy intake and CMD risk factors, we did not further examine the role of the FFAs as potential mediators.
All analyses were performed in R (Version 3.6.3) [50]. Visualizations of the intercorrelations between FFAs was performed using the corrplot R package [51], and visualizations of the associations were performed using the ggplot2 R package [52]. The script for the circular plots were adapted from Ladroue [53]. For all models, the level of significance was set at p ≤ 0.05. However, due to the large number of associations examined in this work, the models were also adjusted for multiple comparisons [48]. Both significant raw and FDR-adjusted p-values are relevant and presented; significant FDR-adjusted results are highlighted where appropriate to help focus the findings.
Results
Population characteristics
The characteristics of the study population are presented in Table 1. The median age of the participants was 60 years, and a majority were highly educated (55%) and non-smokers (69%). Approximately 5% of the participants were following a diet in the month preceding study enrollment. About half (54%) of the participants were overweight or obese, 33% had hypertension, 76% had suboptimal cholesterol levels, and 2% had diabetes. The distribution of the continuous MetS score and SCORE is presented in Fig. S1. Significant differences observed between men and women for several CMD risk parameters (waist circumference, blood pressure, HDL-C, plasma glucose) reflected the patterns observed in the total NQplus population [29].
The median total dairy intake of the study participants was 306 g/day, with dairy fat accounting for approximately 15 g/day (5%) of average daily energy intake. Low-fat dairy products (comprising mostly low-fat FD) were consumed at a higher level than high-fat dairy products (median: 237 vs. 40 g/day), and FD products had a higher level of intake compared to NFD products (median: 151 vs. 88 g/day). No significant differences in dairy intakes were observed between men and women. Among other dietary factors, men had significantly higher intake of total energy and several nutrients (fat, carbohydrates, fiber, protein, sodium) and foods (alcohol, potatoes, meat, sauces, and snack foods) compared to women. Significant differences were also observed for coffee intake between sexes (due to the ranking of the data by the Wilcoxon test), but medians were comparable.
Mean plasma FFA concentrations are presented in Table S3. Seventeen of the original 67 FFAs were not detected in at least a third of participants and thus removed from the analyses (primarily short-chain FFAs that are likely to be metabolized rapidly). Out of the remaining 50 FFAs, the majority were detected in plasma at concentrations of less than 1 mg/L, while five FFAs (C16, C18:1 c9, C18:2 c9c12, C18, and C20:4 c5c8c11c14) were detected at much higher concentrations compared to all other FFAs (12.6–75.3 mg/L). An inter-correlation analysis revealed that a large number of FFAs were significantly positively correlated with each other (Fig. S2).
Free fatty acids associated with dairy intake
Among the 50 FFAs listed in Table S3, 21 were positively correlated with one of the variables of dairy intake (raw p ≤ 0.05) (Table S4). After adjusting for multiple comparisons, moderate correlations for dairy intake and 5 FFAs remained significant, specifically for the intake of dairy fat (C15, C16:1 t9, C16 iso, C17 iso, and C18:2 c9t11 + u; rs = 0.26, 0.32, 0.30, 0.35, and 0.29, respectively) and cheese (C18:2 c9t11 + u; rs = 0.30) (all FDR p ≤ 0.05). Out of the summed groups, summed conjugated linoleic acids (CLA) were also significantly positively correlated with dairy fat (rs = 0.29) and cheese intake (rs = 0.31), and summed C15 and C17, with and without C16:1 t9, was positively associated with total FD intake (rs = 0.26) (FDR p ≤ 0.05).
The correlations between FFAs and dairy intake were largely confirmed in the multiple linear regression models (Tables S5, S6). In the fully adjusted model (Fig. 1), positive associations were observed between 14 individual FFAs and multiple dairy groups/foods (raw p ≤ 0.05; non-significant after FDR adjustment). The strongest associations were observed between the medium-chain unsaturated FFA C16:1 t9 with dairy fat [standardized β (Std. β) = 4.9, R2 = 0.2], high-fat dairy (Std. β = 4.8, R2 = 0.2), high-fat FD (Std. β = 4.9, R2 = 0.2), and cheese (Std. β = 3.5, R2 = 0.1) (raw p ≤ 0.05). Significant associations between C15 with dairy fat (Std. β = 1.0, R2 = 0.1) and high-fat dairy (Std. β = 1.0, R2 = 0.2) intake were also observed albeit with lower effect size (no significant associations were observed for C17).
Summary of the associations between FFAs and dairy intake groups in the fully adjusted model (Model 3). Individual and summed FFAs present in more than a third of participants are included. The direction and magnitude of the standardized regression coefficient (Std. β) is presented as a color gradient where blue indicates positive associations and red indicates negative associations. Significance is emphasized with an asterisk: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. All significant results presented are raw p-values (not significant with FDR adjustment)
Additionally, the long-chain FFAs C17 iso, C22, and C20:3 c8c11c14 were found to be positively associated with the intake of multiple dairy groups/foods; the latter two FFAs were primarily associated with low-fat dairy foods/groups (raw p ≤ 0.05). Among the summed FFA groups, sum of CLA was positively associated with dairy fat intake, while sum of C18:2t with CLA was positively associated with cheese intake. A small number of negative associations were observed between FFAs and primarily low-fat and NFD groups (including milk), namely the long-chain unsaturated FFAs C20:1 c11, C17:1 c10, C18:2 t9t12, C18:3 c6c9c12, C18:1 c9, the medium-chain unsaturated FFA C16:1 u1, in addition to sum of C18:2t without CLA and sum of MUFA.
Free fatty acids associated with cardiometabolic disease risk parameters
The results of the associations between FFAs and CMD risk parameters are presented in Tables S7 and S8. In the fully adjusted model, 33 FFAs were associated with CMD risk parameters, with the majority (30 FFAs) positively associated with increased CMD risk (raw p ≤ 0.05). After adjusting for multiple comparisons, associations for 10 FFAs remained significant, which involved primarily long-chain saturated (C16, C17, C18, and C19) and unsaturated FFAs (C17:1 c10, C18:1 c15, C18:2 c9c12, C20:3 c8c11c14, C20:4 c5c8c11c14, and C22:6 c4c7c10c13c16c19) (FDR p ≤ 0.05) (Fig. 2). These FFAs were positively associated with plasma total and LDL-C, serum triglycerides, and SCORE; the strongest associations were observed for C18:1 c15 with plasma total (Std. β = 6.3, R2 = 0.3) and LDL-C (Std. β = 5.6, R2 = 0.2) (FDR p ≤ 0.05). Several summed FFAs (total, medium-chain, long-chain, omega-3, omega-6, PUFA, saturated, unsaturated, as well as sum of C18:2 and sum of n-C12, n-C14, and n-C16), were also positively associated with plasma total and LDL-C, or SCORE, albeit with a low magnitude of association (Std. β < 0.1, FDR p ≤ 0.05). Sum of C15 and C17, and C15, C17, and C16:1 t9 were also positively associated with total cholesterol (Std. β = 0.8, FDR p ≤ 0.05). FFAs negatively associated with CMD risk parameters included C12:1 u with waist circumference, C20, C18:2 t9t12, C18:4 c6c9c12c15, and C18:1 u1 with serum triglycerides, and C18:1 u1 with plasma LDL-C (raw p ≤ 0.05; non-significant after FDR adjustment) (Fig. S3).
Summary of the associations between FFAs and selected CMD risk factors in the fully adjusted model (Model 3). Individual and summed FFAs present in more than a third of participants are included. The magnitude of the standardized regression coefficients (Std. β) are indicated in each layer of the circle plot, and the direction and significance of the associations are indicated as a color gradient. Significant FDR-adjusted associations are boxed in black. LCFA long-chain fatty acids; LDL low-density lipoprotein; MCFA medium-chain fatty acids; MUFA monounsaturated fatty acids; NS non-significant; PUFA polyunsaturated fatty acids; SFA saturated fatty acids; TG triglycerides; UFA unsaturated fatty acids
Figure 3 presents a summary of the individual and summed FFAs that were significantly associated with both dairy intake and CMD risk parameters. Of these, 5 long-chain unsaturated FFAs (C18:1 t13 + c6 + c7 + u, C18:2 c9t11 + u, C20:1 c11, C20:3 c8c11c14, and C20:4 c5c8c11c14), 2 medium-chain saturated FFAs (C15, C15 iso), and one medium-chain trans FFA (C16:1 t9) were positively associated with at least one variable of dairy intake, as well as plasma total and LDL-C, blood pressure, and SCORE. Interestingly, one long-chain PUFA, C18:2 t9t12, was positively associated with high-fat FD intake but negatively associated with serum triglyceride levels, while one long-chain saturated FFA, C18:1 u1, was positively associated with cheese intake but negatively associated with plasma LDL-C and serum triglyceride levels.
FFAs significantly associated with both dairy intake and CMD risk parameters in the fully-adjusted model (Model 3). Significant positive associations are shown with red circles, and significant negative associations are shown with blue circles, and significant FDR-adjusted associations are outlined in black (p ≤ 0.05). BMI body mass index; DBP, diastolic blood pressure; FD fermented dairy; HDL high-density lipoprotein; LC long-chain; LDL low-density lipoprotein; MC medium-chain; MetS metabolic syndrome; MUFA monounsaturated fatty acid; NFD, non-fermented dairy; PUFA polyunsaturated fatty acid; SFA saturated fatty acid; SBP systolic blood pressure; SCORE Systematic COronary Risk Evaluation
Associations between dairy intake and cardiometabolic health
The results of the associations between dairy intake and CMD risk factors are presented in Tables S9 and S10. Only a small handful of associations were significant, but these associations attenuated in the final adjusted model (model 3), where only an association between total FD intake and low-fat FD intake and SCORE was observed (both: Std. β = 0.2, R2 = 0.2, raw p = 0.04). No associations remained significant after FDR adjustment.
Discussion
In the current study, we performed a thorough examination of the associations between dairy intake, milk-derived FFAs, and CMD risk parameters. We found that 14 medium- and long-chain FFAs were significantly positively associated with self-reported dairy intake, particularly high-fat dairy intake and dairy fat intake. While some of these FFAs are known to be promising FIBs for dairy fat intake (e.g., C15, C16:1 t9), some of the long-chain unsaturated FFAs found to be associated with dairy intake have not been previously reported in the literature. Concurrently, significant associations were observed between 10 of these FFAs and several CMD risk parameters, particularly plasma total cholesterol and serum triglycerides. The magnitude and robustness of these relationships were maintained and were independent of adjustment for a number of demographic, lifestyle, and dietary factors. These observations suggest that these FFAs could serve a dual role as FIBs of dairy intake and in helping to inform the risk for CMD.
Several fatty acids have been previously proposed as FIBs of dairy intake, including C14, C14:1, C15, C17, C17:1, and C16:1 t9 [54, 55]. In particular, the odd-chain fatty acids C15 and C17, which are synthesized by intestinal bacteria in ruminants, have been widely used as indirect measures of dairy fat intake in association studies linking dairy intake to cardiovascular disease, stroke, and type II diabetes [14,15,16,17]. In our study, we found consistent positive associations between C15 with high-fat dairy and dairy fat intake, and C16:1 t9 with high-fat dairy, dairy fat, high-fat FD, and cheese intake (no significant associations were observed for C14, C14:1, C17, or C17:1). Significant associations observed for C18:2 c9t11 + u (a CLA found in animal fats) with dairy fat and cheese intake confirmed previous associations between this fatty acid with dairy fat intake [56]. Conversely, we also observed associations for a number of FFAs that have not previously been considered as FIBs for dairy intake, specifically the branched chain fatty acid C17 iso and the trans fatty acid C18:1 t13 + c6 + c7 + u for high-fat dairy and dairy fat intakes. A few FFAs were also found to be positively associated with low-fat dairy groups (C22) and specific dairy foods (C15 iso, C18:1 u1, C18:2 u3 for cheese, and C20:4 c8c11c14c17 for yoghurt). In addition, although all of the evaluated FFAs have been shown to be present in milk and should theoretically align with (self-reported) dairy intake, we found inverse associations between several FFAs and dairy fat intake, including C16:1 u1, C17:1 c10, C18:3 c6c9c12, and C20:1 c11. This finding illustrates a key conundrum in the identification and validation of FIBs, whereby metabolites found ubiquitously in multiple food sources could challenge their specificity as FIBs for dairy [57]. For instance, C17:1 is known to be a minor constituent of ruminant fats [58], yet the negative association between this fatty acid and dairy fat intake persisted regardless of adjustment for meat intake. To address this, we have recently shown that a multi-marker comprising multiple metabolites (i.e., a combination of a dairy fatty acid, a non-fatty acid dairy metabolite, and a metabolite reflecting physiological characteristics) may produce a more robust assessment of dairy food intake [30]. Nonetheless, identifying which fatty acids are strongly associated with the intake of specific dairy foods is useful for considering their inclusion in multi-marker panels that could help improve the dietary assessment of distinct dairy foods and dairy subgroups.
An important piece of insight gained in the current analysis is the combined effect of analytical method and classification of ‘high-fat’ dairy on the strength of the associations observed. Compared to our previous study where we evaluated the robustness of plasma C15 and C17 as FIBs of dairy intake study in a larger subset of the same population [30], stronger correlations were observed for the same evaluations in the current study. The improved correlations may be partly attributed to the quantitative rather than semi-quantitative approach used for the measurement of plasma free fatty acids. In particular, while we previously observed non-significant correlations between C15 with high-fat dairy and high-fat NFD groups, these correlations were significant and positive in the current study. Additionally, we observed stronger associations between several FFAs and absolute dairy fat intake, rather than high-fat dairy intake. This could be attributed to the method of classification of dairy foods as ‘high-fat’: based on what is considered a high-fat content for each dairy food (as commonly used for dairy food classification) versus based on the total fat content in the dairy food. Thus, careful consideration is needed for the classification of ‘high-fat’ dairy intake in studies where dairy biomarkers are evaluated.
Our study additionally contributes to the current state of the knowledge of using plasma FFAs as reliable biomarkers of habitual dairy fat intake. To date, there have only been a handful of reports investigating FFAs as potential FIBs of dietary fat compared with total fatty acids or fatty acids from other blood fractions, such as cholesteryl esters or serum phospholipids [59, 60]. However, these blood fractions best capture short-term dietary fat intake (past several days or hours), as opposed to long-term intake, which is best captured in erythrocyte membrane or adipose tissue [61, 62]. These biosamples can be difficult to process and store (e.g., hemolysis of erythrocytes from whole blood) or are invasive (in the case of adipose tissue) [63]. As such, they are typically not readily available in biobanks for large population-based studies. In the current report, we demonstrate that plasma FFA can be reliable FIBs of habitual dairy fat intake with associations comparable to studies measuring total FA or FA in various blood fractions.
Aside from their role as FIBs for dairy, milk-derived FFAs could play a dual role in understanding CMD risk. Of all the nutrient groups in dairy, saturated fats have arguably received the most public health attention, due to their adverse effects on circulating blood lipid profile [3, 4]. However, saturated fat is not a homogenous nutrient group, but rather consists of diverse individual fatty acids with distinct functional roles. Subgroups of medium-chain, odd-chain, and very long-chain saturated fatty acids found in dairy have been associated with lower risk of type II diabetes and improved overall metabolic health [64]. Levels of the odd-chain fatty acid C15 in plasma or serum have been linked with a higher risk of ischemic heart disease (in women) [65], but lower risk of developing type II diabetes, myocardial infarction, cardiovascular disease, and coronary heart disease [17, 66,67,68]. Plasma C16:1 t9 has been associated with higher LDL-C, but also with an improved metabolic profile, lower triglycerides, fasting insulin, blood pressure, and incident type II diabetes [18,19,20]. In our study, we observed the same heterogenous effects between individual FFAs with different indicators of CMD risk. Our analyses revealed positive associations for ~ 30 FFAs and negative associations for 5 FFAs with different CMD risk parameters. Notably, 8 FFAs that were also significantly associated with dairy intake (including C15, C16:1 t9) were positively associated with plasma total, LDL-C, serum triglycerides, plasma glucose, blood pressure, continuous MetS, and/or SCORE, while two (C18:2 t9t12, C18:1 u1) were negatively associated with plasma LDL-C and serum triglycerides. These exploratory results suggest that distinct milk-derived FFAs could help inform different aspects of CMD risk and progression. However, causal inferences could not be established (based on the cross-sectional nature of the analyses), and thus further verification of these associations is required.
Further, we did not obtain clear associations between self-reported dairy intake and the CMD risk parameters evaluated. All of the associations were neutral and non-significant, aside from a weak positive association between total and low-fat FD intake and SCORE. The lack of significant associations could reflect the current state of the literature where, collectively, studies examining associations between dairy intake and CMD risk have produced neutral outcomes [69]. Here, the use of FIBs for dairy may better relate to the CMD outcomes by accounting for an aspect of individual variability. Evidently, examining the health impact of a single (dairy) food or food group on CMD also has its limitations, whereas examining their intake in the context of a healthy dietary pattern could offer a stronger explanation of diet-health relations. The lack of associations may also be explained by several study limitations. Our study population was small and relatively healthy, and a larger population with greater variation in CMD risk may have afforded more power to associate the dietary intake of dairy foods to CMD risk parameters. Additionally, based on the data available, we relied on a window of ± 14 days between biosample collection and the completion of an FFQ. This assumes that dietary intakes the day prior to biosample collection were comparable to the reported intakes within the reference range of the FFQ, but otherwise, would be a source of measurement error. The use of cross-sectional data also assumes that the current diet reflects past dietary exposures that are responsible for the current disease risk and furthermore that no major dietary changes have initiated following the appearance of early disease risk markers (e.g., weight gain). Although we had a relatively healthy population, the possibility of reverse causality could not be fully excluded (e.g., awareness of elevated cholesterol prompts changes in diet for some participants). For these reasons, CMD causality could not be assessed. Notwithstanding, a strength of the current study is our comprehensive evaluation of dairy intake related biomarkers with multiple individual and composite CMD risk outcomes, which allows us to see how different FFAs associate with and impact these outcomes separately.
In conclusion, our study examining associations between dairy intake, milk-derived FFAs, and CMD risk parameters resulted in the identification of a panel of 10 medium- and long-chain FFAs that were dually associated with both dairy intake and CMD risk. The inclusion of these FFAs in future multi-marker panels could help improve the dietary assessment of different dairy foods. Further exploration in additional, larger prospective cohorts would allow the potential mediating role of these FFAs between dairy intake and CMD to be assessed, and could help confirm their role in CMD risk pathways.
Data availability
All the data presented in this study can be found in the article and accompanying supplemental files.
Code availability
All packages used in this study are cited and available from publically retrievable sources. The code used in this study is available on request from the corresponding author.
References
Wilkins E, Wilson L, Wickramasinghe K et al (2017) European cardiovascular disease statistics 2017. European Heart Network, Brussels
Yu E, Malik VS, Hu FB (2018) Cardiovascular disease prevention by diet modification: JACC health promotion series. J Am Coll Cardiol 72(8):914–926. https://doi.org/10.1016/j.jacc.2018.02.085
Astrup A (2014) Yogurt and dairy product consumption to prevent cardiometabolic diseases: epidemiologic and experimental studies. Am J Clin Nutr 99(5 Suppl):1235S-S1242. https://doi.org/10.3945/ajcn.113.073015
Turpeinen O (1979) Effect of cholesterol-lowering diet on mortality from coronary heart disease and other causes. Circulation 59(1):1–7. https://doi.org/10.1161/01.CIR.59.1.1
Soedamah-Muthu SS, Verberne LD, Ding EL et al (2012) Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension 60(5):1131–1137. https://doi.org/10.1161/HYPERTENSIONAHA.112.195206
Holmberg S, Thelin A (2013) High dairy fat intake related to less central obesity: a male cohort study with 12 years’ follow-up. Scand J Prim Health Care 31(2):89–94. https://doi.org/10.3109/02813432.2012.757070
Crichton GE, Alkerwi A (2014) Whole-fat dairy food intake is inversely associated with obesity prevalence: findings from the Observation of Cardiovascular Risk Factors in Luxembourg study. Nutr Res 34(11):936–943. https://doi.org/10.1016/j.nutres.2014.07.014
Engel S, Elhauge M, Tholstrup T (2018) Effect of whole milk compared with skimmed milk on fasting blood lipids in healthy adults: a 3-week randomized crossover study. Eur J Clin Nutr 72(2):249–254. https://doi.org/10.1038/s41430-017-0042-5
Duarte C, Boccardi V, Amaro Andrade P et al (2021) Dairy versus other saturated fats source and cardiometabolic risk markers: systematic review of randomized controlled trials. Crit Rev Food Sci Nutr 61(3):450–461. https://doi.org/10.1080/10408398.2020.1736509
Astrup A, Geiker NRW, Magkos F (2019) Effects of full-fat and fermented dairy products on cardiometabolic disease: food is more than the sum of its parts. Adv Nutr 10(5):924S-S930. https://doi.org/10.1093/advances/nmz069
Sluijs I, Forouhi NG, Beulens JW et al (2012) The amount and type of dairy product intake and incident type 2 diabetes: results from the EPIC-InterAct Study. Am J Clin Nutr 96(2):382–390. https://doi.org/10.3945/ajcn.111.021907
Buendia JR, Li Y, Hu FB et al (2018) Regular yogurt intake and risk of cardiovascular disease among hypertensive adults. Am J Hypertens 31(5):557–565. https://doi.org/10.1093/ajh/hpx220
Brouwer-Brolsma EM, Brennan L, Drevon CA et al (2017) Combining traditional dietary assessment methods with novel metabolomics techniques: present efforts by the Food Biomarker Alliance. Proc Nutr Soc 76(4):619–627. https://doi.org/10.1017/S0029665117003949
Chen M, Li Y, Sun Q et al (2016) Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am J Clin Nutr 104(5):1209–1217. https://doi.org/10.3945/ajcn.116.134460
Yakoob MY, Shi P, Willett WC et al (2016) Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation 133(17):1645–1654. https://doi.org/10.1161/CIRCULATIONAHA.115.018410
Yakoob MY, Shi P, Hu FB et al (2014) Circulating biomarkers of dairy fat and risk of incident stroke in U.S. men and women in 2 large prospective cohorts. Am J Clin Nutr 100(6):1437–47. https://doi.org/10.3945/ajcn.114.083097
Santaren ID, Watkins SM, Liese AD et al (2014) Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am J Clin Nutr 100(6):1532–1540. https://doi.org/10.3945/ajcn.114.092544
Mozaffarian D, Cao H, King IB et al (2010) Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med 153(12):790–9. https://doi.org/10.7326/0003-4819-153-12-201012210-00005
Mozaffarian D, Cao H, King IB et al (2010) Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr 92(6):1350–1358. https://doi.org/10.3945/ajcn.110.003970
Mozaffarian D, de Oliveira Otto MC, Lemaitre RN et al (2013) trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 97(4):854–861. https://doi.org/10.3945/ajcn.112.045468
Liu Z, Rochfort S, Cocks B (2018) Milk lipidomics: what we know and what we don’t. Prog Lipid Res 71:70–85. https://doi.org/10.1016/j.plipres.2018.06.002
Mansson HL (2008) Fatty acids in bovine milk fat. Food Nutr Res. https://doi.org/10.3402/fnr.v52i0.1821
Fernandez ML, West KL (2005) Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr 135(9):2075–2078. https://doi.org/10.1093/jn/135.9.2075
Forouhi NG, Koulman A, Sharp SJ et al (2014) Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2(10):810–818. https://doi.org/10.1016/S2213-8587(14)70146-9
Drouin-Chartier JP, Hernandez-Alonso P, Guasch-Ferre M et al (2021) Dairy consumption, plasma metabolites, and risk of type 2 diabetes. Am J Clin Nutr 114(1):163–174. https://doi.org/10.1093/ajcn/nqab047
Airhart S, Cade WT, Jiang H et al (2016) A diet rich in medium-chain fatty acids improves systolic function and alters the lipidomic profile in patients with type 2 diabetes: a pilot study. J Clin Endocrinol Metab 101(2):504–512. https://doi.org/10.1210/jc.2015-3292
Labarthe F, Gelinas R, Des Rosiers C (2008) Medium-chain fatty acids as metabolic therapy in cardiac disease. Cardiovasc Drugs Ther 22(2):97–106. https://doi.org/10.1007/s10557-008-6084-0
Brouwer-Brolsma EM, Streppel MT, van Lee L et al (2017) A national dietary assessment reference database (NDARD) for the Dutch population: rationale behind the design. Nutrients 9(10):1136. https://doi.org/10.3390/nu9101136
Brouwer-Brolsma EM, van Lee L, Streppel MT et al (2018) Nutrition Questionnaires plus (NQplus) study, a prospective study on dietary determinants and cardiometabolic health in Dutch adults. BMJ Open 8(7):e020228. https://doi.org/10.1136/bmjopen-2017-020228
Li KJ, Burton-Pimentel KJ, Brouwer-Brolsma EM et al (2021) Evaluating the robustness of biomarkers of dairy food intake in a free-living population using single- and multi-marker approaches. Metabolites 11(6):395. https://doi.org/10.3390/metabo11060395
The Dutch National Institute for Public Health and the Environment (RIVM) (2011) Nevo-Tabel. Nederlands Voedingsstoffenbestand. Voedingscentrum, Den Haag
Li KJ, Brouwer-Brolsma EM, Burton KJ et al (2020) Prevalence of fermented foods in the Dutch adult diet and validation of a food frequency questionnaire for estimating their intake in the NQplus cohort. BMC Nutr 6(1):69. https://doi.org/10.1186/s40795-020-00394-z
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Mach F, Baigent C, Catapano AL et al (2020) 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 41(1):111–188. https://doi.org/10.1093/eurheartj/ehz455
Williams B, Mancia G, Spiering W et al (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39(33):3021–3104. https://doi.org/10.1093/eurheartj/ehy339
Cosentino F, Grant PJ, Aboyans V et al (2020) 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 41(2):255–323. https://doi.org/10.1093/eurheartj/ehz486
Alberti KG, Eckel RH, Grundy SM et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644
Eisenmann JC (2008) On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol 7:17. https://doi.org/10.1186/1475-2840-7-17
Jung KJ, Jee YH, Jee SH (2017) Metabolic risk score and vascular mortality among Korean adults. Asia Pac J Public Health 29(2):122–131. https://doi.org/10.1177/1010539516688082
DeBoer MD, Gurka MJ (2017) Clinical utility of metabolic syndrome severity scores: considerations for practitioners. Diabetes Metab Syndr Obes 10:65–72. https://doi.org/10.2147/DMSO.S101624
Conroy RM, Pyorala K, Fitzgerald AP et al (2003) Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 24(11):987–1003. https://doi.org/10.1016/s0195-668x(03)00114-3
Piepoli MF, Hoes AW, Agewall S et al (2016) 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 23(11):N1–N96. https://doi.org/10.1093/eurheartj/ehw106
Taylor DH Jr, Hasselblad V, Henley SJ et al (2002) Benefits of smoking cessation for longevity. Am J Public Health 92(6):990–996. https://doi.org/10.2105/ajph.92.6.990
Chinapaw MJ, Slootmaker SM, Schuit AJ et al (2009) Reliability and validity of the Activity Questionnaire for Adults and Adolescents (AQuAA). BMC Med Res Methodol 9:58. https://doi.org/10.1186/1471-2288-9-58
Collomb M, Bütikofer U, Sieber R et al (2002) Composition of fatty acids in cow’s milk fat produced in the lowlands, mountains and highlands of Switzerland using high-resolution gas chromatography. Int Dairy J 12(8):649–659. https://doi.org/10.1016/S0958-6946(02)00061-4
Kim J, Burton-Pimentel KJ, Fleuti C et al (2021) Microbiota and metabolite modifications after dietary exclusion of dairy products and reduced consumption of fermented food in young and older men. Nutrients 13(6):1905. https://doi.org/10.3390/nu13061905
Collomb M, Bühler T (2000) Analyse de la composition en acides gras de la graisse de lait. I. Optimisation et validation d’une méthode générale à haute résolution. Trav Chim Aliment dHygiène 91:306–332
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B 57:289–300
Willett WC (2013) Nutritional epidemiology, 3rd edn. Oxford University Press, New York
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Wei T, Simko V (2021) R package ‘corrplot’: visualization of a correlation matrix (Version 0.92). R Foundation for Statistical Computing, Vienna
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Ladroue C (2012) Polar histogram pretty and useful_Christophe Ladroue 2012. R Foundation for Statistical Computing, Vienna
Biong AS, Berstad P, Pedersen JI (2006) Biomarkers for intake of dairy fat and dairy products. Eur J Lipid Sci Tech 108(10):827–834. https://doi.org/10.1002/ejlt.200600044
Münger LH, Garcia-Aloy M, Vazquez-Fresno R et al (2018) Biomarker of food intake for assessing the consumption of dairy and egg products. Genes Nutr 13:26. https://doi.org/10.1186/s12263-018-0615-5
Pranger IG, Corpeleijn E, Muskiet FAJ et al (2019) Circulating fatty acids as biomarkers of dairy fat intake: data from the lifelines biobank and cohort study. Biomarkers 24(4):360–372. https://doi.org/10.1080/1354750X.2019.1583770
Ratnayake WM (2015) Concerns about the use of 15:0, 17:0, and trans-16:1n–7 as biomarkers of dairy fat intake in recent observational studies that suggest beneficial effects of dairy food on incidence of diabetes and stroke. Am J Clin Nutr 101(5):1102–1103. https://doi.org/10.3945/ajcn.114.105379
Alves SP, Marcelino C, Portugal PV, Bessa RJB (2006) Short communication : the nature of heptadecenoic acid in ruminant fats. J Dairy Sci 89(1):170–173. https://doi.org/10.3168/jds.S0022-0302(06)72081-1
Azab SM, de Souza RJ, Teo KK et al (2020) Serum nonesterified fatty acids have utility as dietary biomarkers of fat intake from fish, fish oil, and dairy in women. J Lipid Res 61(6):933–944. https://doi.org/10.1194/jlr.D120000630
Hodson L, Skeaff CM, Fielding BA (2008) Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47(5):348–380. https://doi.org/10.1016/j.plipres.2008.03.003
Arab L (2003) Biomarkers of fat and fatty acid intake. J Nutr 133(3):925S–32S. https://doi.org/10.1093/jn/133.3.925S
Baylin A, Campos H (2006) The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol 17(1):22–27. https://doi.org/10.1097/01.mol.0000199814.46720.83
Brenna JT, Plourde M, Stark KD et al (2018) Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. Am J Clin Nutr 108(2):211–227. https://doi.org/10.1093/ajcn/nqy089
Guo J, Givens DI, Astrup A et al (2019) The impact of dairy products in the development of type 2 diabetes: where does the evidence stand in 2019? Adv Nutr 10(6):1066–1075. https://doi.org/10.1093/advances/nmz050
Sun Q, Ma J, Campos H, Hu FB (2007) Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr 86(4):929–937. https://doi.org/10.1093/ajcn/86.4.929
Warensjo E, Jansson JH, Cederholm T et al (2010) Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case-control study. Am J Clin Nutr 92(1):194–202. https://doi.org/10.3945/ajcn.2009.29054
Aslibekyan S, Campos H, Baylin A (2012) Biomarkers of dairy intake and the risk of heart disease. Nutr Metab Cardiovasc 22(12):1039–1045. https://doi.org/10.1016/j.numecd.2011.02.003
de Oliveira Otto MC, Nettleton JA, Lemaitre RN et al (2013) Biomarkers of dairy fatty acids and risk of cardiovascular disease in the Multi-ethnic Study of Atherosclerosis. J Am Heart Assoc 2(4):e000092. https://doi.org/10.1161/JAHA.113.000092
Poppitt SD (2020) Cow’s milk and dairy consumption: is there now consensus for cardiometabolic health? Front Nutr 7:574725. https://doi.org/10.3389/fnut.2020.574725
Acknowledgements
We thank all NQplus participants for their valuable contribution to this study and their cooperation. We would also like to thank the dedicated research staff that was involved in execution of this study. We would like to give a special thanks to the dieticians of the Division of Human Nutrition at Wageningen University in Wageningen, the Netherlands. We would also like to thank Veiligheids-en Gezondheids Regio Gelderland-midden (Arnhem, The Netherlands) for their help with recruitment. We thank Marion Buso for providing some scripts for the spline regression analysis, and to Monika Spahni for helping to measure some plasma samples.
Funding
Funding to conduct the targeted free fatty acid analyses was provided by Agroscope, the Federal Department of Economic Affairs, Education and Research (EAER), Federal Office for Agriculture (FOAG) (Bern, Switzerland) for the Cardioferment Project. NDARD/NQplus was core funded by ZonMw (ZonMw, Grant 91110030). NDARD/NQPlus were also supported by Wageningen University (Wageningen, the Netherlands); add-on funding ZonMW Gezonde Voeding DHD-index (ZonMw, Grant 115100007); add-on validation of BBMRI FFQ and Maastricht FFQ (Grant BBMRI-NL RP9 and CP2011-38).
Author information
Authors and Affiliations
Contributions
KJB-P, EMB-B, EJMF and GV were involved in the conceptualization of the study and in project administration. KJL performed the dietary and association analyses. The targeted free fatty acid analyses were performed by CF and MS, and supervised by RB. KJL wrote the initial draft with input from EMB-B and KJB-P. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Wageningen University & Research (Protocol Number NL34775.081.10 and date of approval 11-07-2011).
Consent to participate
Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, K.J., Brouwer-Brolsma, E.M., Fleuti, C. et al. Associations between dairy fat intake, milk-derived free fatty acids, and cardiometabolic risk in Dutch adults. Eur J Nutr 62, 185–198 (2023). https://doi.org/10.1007/s00394-022-02974-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02974-0