Abstract
Purpose
Low-grade inflammation in obesity is associated with insulin resistance and other metabolic disturbances. In response to high-energy meal intake, blood concentrations of inflammatory markers, glucose and insulin rise. The aim of this study was to examine whether a basal inflammatory state influences postprandial responses.
Methods
A randomized crossover trial was performed in 60 participants with a cardiometabolic risk phenotype (age 70 ± 5 years; BMI 30.9 ± 3.1 kg/m2). Each participant consumed three different iso-energetic meals (4300 kJ): a Western diet-like high-fat meal (WDHF), a Western diet-like high-carbohydrate meal (WDHC) and a Mediterranean diet-like meal (MED). Blood samples were collected when fasted and hourly for 5 h postprandially and analyzed for glucose, insulin, interleukin-1β (IL-1β), interleukin-6 (IL-6) and endothelial adhesion molecules. Based on fasting serum C-reactive protein (CRP) concentrations, participants were assigned to a high inflammation (CRP ≥ 2.0 mg/L; n = 30) or low inflammation (CRP < 2.0 mg/L; n = 30) group, and postprandial outcomes were compared.
Results
Plasma IL-6, glucose and serum insulin increased after all meals, while IL-1β and endothelial adhesion molecules were unchanged. The high inflammation group had higher fasting and postprandial IL-6 concentrations than the low inflammation group, although the IL-6 response slope was similar between groups. In response to the WDHC meal, participants in the high inflammation group experienced a higher glycaemic response than those in the low inflammation group.
Conclusion
A basal proinflammatory state results in higher absolute fasting and postprandial IL-6 concentrations, but the increase in IL-6 relative to basal levels is not different between high and low inflammation groups. Elevated glycaemic response in the high inflammation group may be due to inflammation-induced short-term insulin resistance.
The trial was registered at http://www.germanctr.de and http://www.drks.de under identifier DRKS00009861 (registration date, January 22, 2016).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-grade systemic inflammation (‘metaflammation’) is a characteristic of the obese state since adipose tissue releases a wide range of inflammatory mediators [1, 2]. The source of inflammatory mediators within adipose tissue is not fully elucidated: although adipocytes themselves play a role, infiltrating macrophages are also important [1]. Obese adults have higher circulating concentrations of inflammatory markers than lean adults (measured in the “resting state”, e.g., fasting), and these are believed to play a role in the development of insulin resistance and other metabolic disturbances of the metabolic syndrome and type 2 diabetes [1]. Blood concentrations of inflammatory markers are lowered following energy restriction and fat mass loss [1, 3]. In the hours following consumption of a high-energy meal, there is an elevation in circulating inflammatory mediator concentration, which is exaggerated in obese and type 2 diabetic subjects [1]. Evidence suggests that repeated exposure to postprandial inflammation stresses metabolic adaptation systems, leading to a dysmetabolic state [2]. In addition, the postprandial inflammation is closely associated with endothelial activation, indicated by the increased expression of endothelial adhesion molecules [4].
Recently, we reported that intake of energy-rich meals (~ 4300 kJ/meal) resulted in glycemia, lipemia and an inflammatory response in populations of older adults with a risk phenotype for cardiometabolic diseases [5, 6]. A meal typical of the Mediterranean diet resulted in favorable effects on glycemic, insulinemic and lipemic responses compared with two meals typical of Western dietary patterns [5]. However, as the inflammatory response was present to the same degree after each of the three iso-energetic meals, we suggested that energy intake is the main predictor of postprandial inflammation [5]. However, to the best of our knowledge, there are no studies investigating the influence of baseline inflammatory state on the inflammatory response to meal intake in older adults with metabolic syndrome traits. Thus, the aim of the present secondary analysis of data of our previous postprandial study [5] was to investigate whether a basal, low-grade inflammatory state would influence inflammatory responsiveness to the intake of different high-energetic meal patterns.
Subjects and methods
Subjects and study design
Participants were recruited in Bonn, Germany, via advertisements in local newspapers, public posting and flyers. Details of the study design and subject recruitment, enrollment and randomization have been described previously [5]. In brief, interested volunteers (n = 127) aged 60–80 years with a BMI > 27 kg/m2 attended screening, which included physical assessments, laboratory assessments, medical history and a dietary questionnaire. Overweight or obese participants were included if they had the following traits of metabolic syndrome: (1) visceral fat distribution (waist circumference ≥ 94 cm for men and ≥ 80 cm for women); (2) pre-hypertension (systolic BP ≥ 120–139 mmHg and/or diastolic BP ≥ 80–89 mmHg) or hypertension (systolic BP ≥ 140–159 mmHg and/or diastolic BP ≥ 90–99 mmHg); and (3) at least one of the following criteria: impaired glucose tolerance (fasting plasma glucose ≥ 5.55 mmol/L) and/or dyslipidemia (fasting serum triglycerides ≥ 1.7 mmol/L or serum HDL cholesterol < 1.0 mmol/L for men and < 1.3 mmol/L for women) and/or a proinflammatory state (high-sensitivity C-reactive protein ≥ 2.0 mg/dL) [5]. A total of 60 subjects (34 males, 26 females) were included in the study. All subjects completed the trial, and their respective data were included in the analysis. All study procedures were approved by the ethics committee of the Medical Faculty of the University of Bonn, Germany. Written informed consent was obtained from all subjects prior to inclusion. This trial was registered at http://www.germanctr.de and http://drks.de under identifier DRKS00009861 (registration date, January 22, 2016). The participants (n = 60) [5] were retrospectively divided into high inflammation and low inflammation groups and data were reanalysed.
The postprandial study was conducted as a randomized controlled crossover trial. Individuals participated in three, 5 h meal tests. Participants were assigned to the three different test meals by block randomization. Venous blood sampling was conducted prior to the test meal (0 h) and at 1, 2, 3, 4 and 5 h after finishing the test meal. Participants consumed an iso-energetic and iso-nitrogen test meal at each visit. The test meals were representative of real-life dietary intake: a Western diet-like high-fat (WDHF) meal (rich in total fat, SFA, and animal protein), a Western diet-like high-carbohydrate (WDHC) meal (rich in refined carbohydrates) and a Mediterranean diet-like (MED) meal (rich in unsaturated fatty acids, dietary fiber, and antioxidants). The energy and macronutrient composition of the meals are shown in Table 1, and detailed descriptions of meal composition are presented in Schönknecht et al. [5]. The test meals were consumed in the morning as the first meal of the day and had to be ingested within 20 min.
Blood sample processing and analysis
Details of the pre-analytical procedures of fasting and postprandial blood samples have been described previously [5]. Serum total cholesterol was measured using polychromatic endpoint measurement, whereas serum HDL cholesterol, LDL cholesterol and triglycerides were assessed using endpoint measurement with a Dimension Vista 1500 analyser (Siemens Healthcare Diagnostics). Plasma glucose was assessed via bichromatic endpoint measurement (Dimension Vista 1500 analyser), and serum insulin was determined using chemiluminescent-immunometric assay (Immulite 2000 analyzer, Siemens Healthcare Diagnostics). Serum CRP (high sensitive, hs) was determined using a turbidimetric immunoassay (Cobas 8000 modular analyser series). Hs-interleukin-1β (IL-1β) and hs-interleukin-6 (IL-6), soluble adhesion molecules E-selectin (sE-selectin), intercellular adhesion molecule-1 (sICAM-1) and vascular cell adhesion molecule-1 (sVCAM-1) were analyzed using commercially available enzyme-linked immunoassay kits (R&D Systems) [5].
Statistical analysis
Based on CRP assessment at screening visit, participants were divided into (i) high inflammation (defined as fasting serum CRP ≥ 2 mg/L) and (ii) low inflammation (fasting CRP < 2 mg/L) groups [7, 8]. Postprandial responses were analyzed and compared between both groups using SPSS statistical software package (version 23; IBM). Proinflammatory state-related differences between the baseline characteristics at screening were analyzed using unpaired Student’s t tests. Statistical significance was defined as P < 0.05. Prior to analyses, the glucose, insulin and hs-CRP data were log-transformed.
Postprandial outcomes were tested for effects of inflammatory state, time and test meal by performing linear mixed models. Fasting values were included as covariates, and participants were included as random factors. When significant interactions were absent (P > 0.05), mixed model calculations were repeated without the interaction terms. Differences between fasting variables were tested using a mixed model analysis. Residuals obtained from the calculations were inspected for normality to control for the fit of the statistical model. Logarithmic transformation was applied before analysis if the residuals were not normally distributed, which was true for glucose, insulin, IL-6, sICAM-1 and sVCAM-1 data.
The incremental AUC (iAUC) was calculated for the concentration of all postprandial parameters as a summary variable. Calculations were performed as reported previously [5]. Data are expressed as mean ± SEM, and all P values listed are two tailed.
Results
Baseline characteristics
Baseline characteristics are shown in Table 2. Of the 60 participants studied, 30 were assigned to the high inflammation group (10 males, 20 females), and 30 were assigned to the low inflammation group (24 males, 6 females). Age, BMI, waist circumference, fat mass, systolic blood pressure, glucose, insulin and serum lipids were comparable between groups. Significantly higher diastolic blood pressure was evident in the high inflammation group compared with the low inflammation group (P = 0.033).
Postprandial response of IL-6, IL-1β and endothelial adhesion molecules
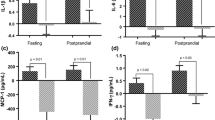
A proinflammatory state had a significant effect on inflammatory responses to test meal intake (Fig. 1). Participants from the high inflammation group showed higher fasting and postprandial IL-6 concentrations than the low inflammation group (P = 0.001). However, increases in IL-6 concentration over the postprandial period were similar between the high and low inflammation groups: in both groups, plasma IL-6 increased by approximately 100% (low inflammation group, 103%; high inflammation group, 100%) from baseline in response to meal intake (Fig. 1).
Fasting and postprandial plasma concentrations of IL-1β (A) and IL-6 (B) for high and low inflammation groups. + + + P < 0.001 for time effect. *P < 0.05; **P < 0.01 for group effect. IL-1β; interleukin-1β; IL-6, interleukin-6; MED, Mediterranean diet-like meal; WDHC, Western diet-like high-carbohydrate meal; WDHF, Western diet-like high-fat meal
Fasting and postprandial concentrations of IL-1β were significantly higher in the high inflammation group than in the low inflammation group (P = 0.005). However, concentrations of IL-1β did not significantly change over time. No differences in fasting or postprandial concentrations of sICAM-1, sVCAM-1 and sE-selectin between the high and low inflammation groups were observed; endothelial adhesion molecules also did not significantly change over time (data not shown). Summary variables (iAUC) for IL-6, IL-1β and endothelial adhesion molecules were not affected by inflammatory status (Table 3).
Postprandial responses of glucose and insulin
The inflammatory state had a meal-dependent effect on glycaemic responses: the high inflammation group had significantly higher postprandial glucose concentrations 2–5 h after the WDHC meal than the low inflammation group (P = 0.006) (Fig. 2). Glucose iAUC values also tended to be higher in the high inflammation group than in the low inflammation group (P = 0.096) (Table 3). Glucose responses to the MED and WDHF meals were not affected by inflammatory state (kinetic data not shown). No differences were observed in insulin responses between the high and low inflammation groups (data not shown).
Discussion
The present report is a secondary analysis using data from the Diet–Body–Brain (DietBB) postprandial study conducted in 60 older individuals with a cluster of metabolic syndrome traits [5]. We found that the relative magnitude of the inflammatory response to meal intake, measured by changes in plasma IL-6 concentrations over a 5-h period, was similar between subjects with and without proinflammatory markers. In addition, glucose response to a WDHC meal was higher and more prolonged in participants in a proinflammatory state.
Our data indicate that relative postprandial inflammatory response intensity is not related to fasting inflammatory status in overweight/obese subjects with metabolic syndrome traits. The current literature on the impact of meals on inflammatory outcomes according to basal inflammatory status is extremely limited. Phillips et al. [9] assessed the effect of a high-fat meal on markers of inflammation in (i) lean, (ii) obese non-diabetic and (iii) type 2 diabetic men. In accordance with our data, obese non-diabetic men presented an inflammatory state, indicated by higher fasting hs-CRP concentrations than lean men (0.2 vs. 1.3 mg/L). Absolute concentrations of IL-6 following the high-fat meal increased in all groups, but there were no differences in response slopes relative to fasting values between groups. In contrast to our study, baseline IL-6 concentrations did not differ between groups [9]; however, the study was limited to males, baseline characteristics (e.g., age, BMI, body composition) differed between groups, and the sample size was small (n = 10 per group).
Another study compared postprandial vascular, inflammatory and leukocyte adherence responses between lean and obese men in an inflammatory state after high-fat milkshakes [10]. No differences in postprandial responses between lean and obese participants were observed. Likewise, Schwander et al. [11] challenged 19 normal-weight and 18 obese men with 2100, 4300 and 6300 kJ high-fat meals. Fasting concentrations of CRP (0.9 ± 0.9 vs. 3.0 ± 3.0 mg/L), but not IL-6, differed between groups. None of the energy doses led to the identification of a significant difference in the postprandial response of inflammatory markers between normal-weight and obese participants. These data confirm that the higher absolute postprandial IL-6 concentrations we observed resulted from an overweight/obesity-induced basal inflammatory state, rather than an amplified inflammatory response. The functions and mechanism of action of IL-6 are complex, meaning that elevated IL-6 concentrations in the basal state do not necessarily result in an augmented postprandial inflammatory response. In addition, the metabolic effects of IL-6 depend on the cellular environment and the concentration [12].
Interleukin-6 is the most frequently assessed marker of inflammation in the postprandial period and consistently increases between 4 and 8 h after intake of a high-fat meal [13]. Elevated IL-6 concentrations have been linked to multiple clinical parameters (e.g., blood pressure, insulin sensitivity and coronary artery disease) [13]. Furthermore, proinflammatory cytokines, such as IL-6 and tumor necrosis factor-α, have been implicated in the progression of atherosclerosis [14] and type 2 diabetes [15, 16]. Although more research is needed regarding the clinical relevance of short-term IL-6 fluctuations after meal intake, one may speculate that over the course of a day, accumulation of IL-6 resulting from repeated meal intake may trigger metabolic dysmetabolism, atherosclerosis and diabetes progression.
Our data also indicate that basal inflammatory state influences postprandial glucose response. Participants from the high inflammation group had higher glucose concentrations over the postprandial period than the low inflammation group. Interestingly, this finding was only observed after WDHC meal intake, not after WDHF and MED meal intake, indicating that the inflammation-related impairment of the glycaemic response may be associated with the amount of carbohydrate consumed. These group differences may be reasoned by the association of low-grade inflammation with insulin resistance [17,18,19]. The subjects in the high-inflammatory group show elevated concentrations of inflammation markers (hs-CRP and IL-6), with the consequence of postprandially impaired insulin sensitivity and subsequently decreased glucose uptake. The repeated exposure to postprandial increased glycemia represents the contribution of low-grade inflammation to insulin resistance.
One mechanism potentially linking the higher glycaemic response in participants from the high inflammation group is the interference by cytokines in insulin action. Proinflammatory signaling molecules activate certain inflammatory pathways, which interfere with intracellular insulin-receptor signaling by activating serine kinases, directly blocking insulin action [1, 2, 18]. Chronically elevated IL-6 concentrations in the basal inflammatory state are associated with substantial metabolic effects, e.g., systemic insulin resistance [19]. In vitro and in vivo models show that insulin action is inhibited in adipocytes, hepatocytes and endothelial cells exposed to cytokines (IL-1β, IL-6 and tumor necrosis factor-α) [20] and other proinflammatory mediators [21,22,23].
To the best of our knowledge, the current study is the first to report that, relative to baseline levels, inflammatory responses to meal intake are similar between subjects with and without a proinflammatory phenotype. One strength of our study is that we focused on a clinically relevant population, i.e., overweight/obese older individuals, and that we used a whole-diet approach to study acute effects of meals on several cardiometabolic parameters [5]. In addition, retrospective division of the 60 participants into high and low inflammation groups based on baseline CRP concentrations resulted in evenly distributed (n = 30 per group) groups with similar anthropometric and metabolic characteristics. Furthermore, assessment over 5 h is adequate to cover most postprandial responses, and parameters were measured hourly, allowing in-depth evaluation of postprandial kinetics.
One limitation of the present work is that it is an exploratory, and thus secondary, data analysis. The study was originally designed to investigate the effects of three different meal patterns on postprandial lipemic, glycemic and inflammatory responses; thus, the presented data are ancillary examinations. In addition, we only selected plasma/serum markers (cytokines and adhesion markers) to characterize the inflammatory response. Currently, no consensus exists regarding inflammatory mediators that best represent low-grade inflammation [1, 24]. In addition, we are far from a consensus regarding response features (i.e., timing and magnitude) after a meal of even the most commonly assessed markers of inflammation [13]. Future postprandial studies should include further biomarkers, such as the examination of immune cell-activation of inflammatory gene expression profiles or inflammatory omic responses [24]. Also, further biomarkers, such as galectin-3, may be considered in postprandial studies to examine the relation between low-grade inflammation and insulin resistance [19]
In conclusion, our data indicate that individuals in a proinflammatory state have higher absolute cytokine blood concentrations postprandially than individuals without elevated proinflammatory markers. The magnitude of the inflammatory response relative to baseline levels was similar between groups, demonstrated by IL-6 concentrations increasing equally for individuals in the high and low inflammation groups. In addition, the glucose response to a WDHC meal was higher and more prolonged in participants in a proinflammatory state. These data suggest that low-grade inflammation may contribute to short-term insulin signaling impairment during the postprandial period, thereby leading to augmented postprandial glucose responses.
Abbreviations
- BP:
-
Blood pressure
- hs-CRP:
-
High-sensitivity C-reactive protein
- EN %:
-
Energy percent
- iAUC:
-
Incremental area under the curve
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- MED:
-
Mediterranean diet-like meal
- sE-selectin:
-
Soluble E-selectin
- sICAM-1:
-
Soluble intercellular adhesion molecule-1
- sVCAM-1:
-
Soluble vascular cell adhesion molecule-1
- WDHF:
-
Western diet-like high-fat meal
- WDHC:
-
Western diet-like high-carbohydrate meal
References
Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jonsson LS, Kolb H, Lansink M, Marcos A, Margioris A, Matusheski N, Nordmann H, O’Brien J, Pugliese G, Rizkalla S, Schalkwijk C, Tuomilehto J, Warnberg J, Watzl B, Winklhofer-Roob BM (2011) Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr 106(Suppl 3):S5-78. https://doi.org/10.1017/S0007114511005460[pii]
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445. https://doi.org/10.1146/annurev-immunol-031210-101322
Egert S, Baxheinrich A, Lee-Barkey YH, Tschoepe D, Wahrburg U, Stratmann B (2014) Effects of an energy-restricted diet rich in plant-derived alpha-linolenic acid on systemic inflammation and endothelial function in overweight-to-obese patients with metabolic syndrome traits. Br J Nutr 112(8):1315–1322. https://doi.org/10.1017/S0007114514002001[pii]
Lacroix S, Rosiers CD, Tardif JC, Nigam A (2012) The role of oxidative stress in postprandial endothelial dysfunction. Nutr Res Rev 25(2):288–301
Schonknecht YB, Crommen S, Stoffel-Wagner B, Coenen M, Fimmers R, Holst JJ, Simon MC, Stehle P, Egert S (2020) Acute effects of three different meal patterns on postprandial metabolism in older individuals with a risk phenotype for cardiometabolic diseases: a randomized controlled crossover trial. Mol Nutr Food Res 64(9):e1901035. https://doi.org/10.1002/mnfr.201901035
Diekmann C, Huber H, Preuss M, Preuss P, Predel HG, Stoffel-Wagner B, Fimmers R, Stehle P, Egert S (2019) Moderate postmeal walking has no beneficial effects over resting on postprandial lipemia, glycemia, insulinemia, and selected oxidative and inflammatory parameters in older adults with a cardiovascular disease risk phenotype: a randomized crossover trial. J Nutr 149(11):1930–1941. https://doi.org/10.1093/jn/nxz148
Brüll V, Burak C, Stoffel-Wagner B, Wolffram S, Nickenig G, Muller C, Langguth P, Alteheld B, Fimmers R, Naaf S, Zimmermann BF, Stehle P, Egert S (2015) Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: a randomised double-blinded placebo-controlled cross-over trial. Br J Nutr 114(8):1263–1277. https://doi.org/10.1017/S0007114515002950[pii]
Bogaty P, Dagenais GR, Joseph L, Boyer L, Leblanc A, Belisle P, Brophy JM (2013) Time variability of C-reactive protein: implications for clinical risk stratification. PLoS One 8(4):e60759. https://doi.org/10.1371/journal.pone.0060759
Phillips LK, Peake JM, Zhang X, Hickman IJ, Briskey DR, Huang BE, Simpson P, Li SH, Whitehead JP, Martin JH, Prins JB (2013) Postprandial total and HMW adiponectin following a high-fat meal in lean, obese and diabetic men. Eur J Clin Nutr 67(4):377–384. https://doi.org/10.1038/ejcn.2013.49
Esser D, van Dijk SJ, Oosterink E, Muller M, Afman LA (2013) A high-fat SFA, MUFA, or n3 PUFA challenge affects the vascular response and initiates an activated state of cellular adherence in lean and obese middle-aged men. J Nutr 143(6):843–851. https://doi.org/10.3945/jn.113.174540
Schwander F, Kopf-Bolanz KA, Buri C, Portmann R, Egger L, Chollet M, McTernan PG, Piya MK, Gijs MA, Vionnet N, Pralong F, Laederach K, Vergeres G (2014) A dose-response strategy reveals differences between normal-weight and obese men in their metabolic and inflammatory responses to a high-fat meal. J Nutr 144(10):1517–1523. https://doi.org/10.3945/jn.114.193565
Hotamisligil GS (2017) Inflammation, metaflammation and immunometabolic disorders. Nature 542(7640):177–185. https://doi.org/10.1038/nature21363
Emerson SR, Kurti SP, Harms CA, Haub MD, Melgarejo T, Logan C, Rosenkranz SK (2017) Magnitude and timing of the postprandial inflammatory response to a high-fat meal in healthy adults: a systematic review. Adv Nutr 8(2):213–225. https://doi.org/10.3945/an.116.014431
Oikonomou E, Leopoulou M, Theofilis P, Antonopoulos AS, Siasos G, Latsios G, Mystakidi VC, Antoniades C, Tousoulis D (2020) A link between inflammation and thrombosis in atherosclerotic cardiovascular diseases: clinical and therapeutic implications. Atherosclerosis 309:16–26. https://doi.org/10.1016/j.atherosclerosis.2020.07.027
Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P, Liu LG (2013) Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36(1):166–175. https://doi.org/10.2337/dc12-0702
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286(3):327–334. https://doi.org/10.1001/jama.286.3.327
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116(7):1793–1801. https://doi.org/10.1172/JCI29069
Saltiel AR, Olefsky JM (2017) Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 127(1):1–4. https://doi.org/10.1172/JCI92035
Lee YS, Olefsky J (2021) Chronic tissue inflammation and metabolic disease. Genes Dev 35(5–6):307–328. https://doi.org/10.1101/gad.346312.120
de Luca C, Olefsky JM (2008) Inflammation and insulin resistance. FEBS Lett 582(1):97–105. https://doi.org/10.1016/j.febslet.2007.11.057
Wu H, Ballantyne CM (2017) Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest 127(1):43–54. https://doi.org/10.1172/JCI88880
Li P, Oh DY, Bandyopadhyay G, Lagakos WS, Talukdar S, Osborn O, Johnson A, Chung H, Maris M, Ofrecio JM, Taguchi S, Lu M, Olefsky JM (2015) LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med 21(3):239–247. https://doi.org/10.1038/nm.3800
Li P, Liu S, Lu M, Bandyopadhyay G, Oh D, Imamura T, Johnson AMF, Sears D, Shen Z, Cui B, Kong L, Hou S, Liang X, Iovino S, Watkins SM, Ying W, Osborn O, Wollam J, Brenner M, Olefsky JM (2016) Hematopoietic-derived galectin-3 causes cellular and systemic insulin resistance. Cell 167(4):973-984 e912. https://doi.org/10.1016/j.cell.2016.10.025
Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, McArdle HJ, Kremer BH, Sterkman L, Vafeiadou K, Benedetti MM, Williams CM, Calder PC (2015) Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. https://doi.org/10.1017/S0007114515002093
Acknowledgements
The authors would like to thank Christine Bierschbach, Anke Carstensen, Anke Ernst, Hedwig Heuser, Christine Laurini, Adelheid Fürste and Anja Stratmann for their excellent technical assistance, and Karoline Maximiliane Bauer, Benjamin Grote, Elena-Katharina Kapitza, Bahrah Ghasi Nouri and Sarina Thelens for performing the venipunctures. The study was supported by the German Federal Ministry of Education and Research (BMBF) within the project “Diet-Body-Brain” (grant no. 01EA1372D).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schönknecht, Y.B., Crommen, S., Stoffel-Wagner, B. et al. Influence of a proinflammatory state on postprandial outcomes in elderly subjects with a risk phenotype for cardiometabolic diseases. Eur J Nutr 61, 3077–3083 (2022). https://doi.org/10.1007/s00394-022-02870-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02870-7