Abstract
Purpose
N-6 polyunsaturated fatty acids (PUFA), particularly linoleic acid (LA), have been associated with lower risk of coronary heart disease (CHD), but little is known about their antiarrhythmic properties. We investigated the association of the serum n-6 PUFAs with the risk of atrial fibrillation (AF), the most common type of cardiac arrhythmia.
Methods
The study included 2450 men from the Kuopio Ischaemic Heart Disease Risk Factor Study, aged 42–60 years at baseline. The total n-6 PUFA includes linoleic acid (LA), arachidonic acid (AA), γ-linolenic acid (GLA) and dihomo-γ-linolenic acid (DGLA). Cox proportional hazards regression was used to estimate hazard ratio (HR) of incident events.
Results
During the mean follow-up of 22.4 years, 486 AF cases occurred. The multivariable-adjusted HR in the highest versus the lowest quartile of total serum n-6 PUFA concentration was 0.79 (95% CI 0.58–1.08, P trend = 0.04). When evaluated individually, only serum LA concentration was inversely associated with AF risk (multivariable-adjusted extreme-quartile HR 0.69, 95% CI 0.51–0.94, P trend = 0.02). These associations were stronger among the men without history of CHD or congestive heart failure at baseline, compared to men with such disease history (P for interaction = 0.05 for total n-6 PUFA and LA). Similar associations were observed with dietary LA and AA intakes. No significant associations were observed with serum AA, GLA or DGLA concentrations.
Conclusions
Higher circulating concentration and dietary intake of n-6 PUFA, mainly LA, are associated with lower risk of AF, especially among men without history of CHD or congestive heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is the most common of all sustained cardiac arrhythmias, especially in elderly [1]. AF is associated with fatigue, reduced exercise tolerance, and increased risk of cardiovascular disease (CVD), including congestive heart failure and stroke, as well as total and CVD mortality [2].
The n-6 polyunsaturated fatty acids (PUFA), especially linoleic acid (LA, 18:2n-6), have been shown to be beneficial regarding cardiovascular health [3, 4]. LA, the predominant n-6 PUFA, is an essential fatty acid and it is found mainly in vegetable oils, nuts, and oily seeds [5]. LA can be endogenously converted to γ-linolenic acid (GLA, 18:3n-6), dihomo-γ-linolenic acid (DGLA; 20:3n-6) and arachidonic acid (AA, 20:4n-6) by stepwise desaturation and chain elongation. These fatty acids, except for DGLA, are also obtained from vegetable oils and animal products such as poultry, meat, fish, seafood, and eggs [5, 6].
It has been indicated that higher intake of LA is associated with lower risk of CVD [4, 7, 8]. Knowledge regarding the cardiovascular effects of AA, GLA and DGLA are still less established. However, few studies have investigated the impact of the n-6 PUFA on the risk of AF, with inconsistent results. One prospective cohort study found an inverse association between plasma AA concentrations and risk of AF [9], whereas a Mendelian randomization study found a suggestive inverse association between genetically-predicted LA concentrations and risk of AF. In contrast, in a prospective cohort study dietary LA or AA intakes were not associated with the risk of AF [11], but higher adipose tissue n-6 PUFA concentration (sum of GLA, DGLA and AA) was associated with lower risk of developing AF [12]. However, LA was associated with an increased risk of AF in men, but not in women [12].
Therefore, because of the limited and inconsistent findings, we investigated the prospective associations of the serum n-6 PUFA concentrations and dietary n-6 PUFA intakes with the risk of AF among middle-aged and older men from the population-based KIHD cohort. We have previously shown in the KIHD cohort that higher n-6 PUFA intake or serum concentrations, especially LA, were associated with lower risk of fatal myocardial infarction, CVD death and total mortality [13, 14]. In addition, as presence of CHD and congestive heart failure is strongly associated with the new onset of AF [15], we investigated and compared the associations of the n-6 PUFAs with AF among participants with and without history of coronary heart disease (CHD) and congestive heart failure at the baseline.
Methods
Study population
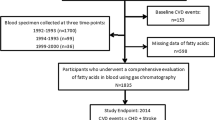
The KIHD is a population-based study designed to investigate risk factors for CVD, atherosclerosis, and related outcomes in men from eastern Finland [16], a total of 2682 men (82.9% of those eligible) who were 42, 48, 54 or 60 years old and living in the city of Kuopio or its surrounding areas participated in the baseline examinations in 1984–1989. The baseline characteristics of the entire study population have been described previously [17]. The KIHD protocol was approved by the Research Ethics Committee of the University of Kuopio and complies with Declaration of Helsinki (ClinicalTrials.gov Identifier: NCT03221127). All the subjects signed a written informed consent. Study participants were not involved in the design, or conduct, or reporting, or dissemination plans of the current study. The manuscript complies with the STROBE checklist. Subjects with a history of AF at baseline (n = 32) were excluded from the analyses. We also excluded men with missing data on serum n-6 PUFAs (n = 200), leaving 2450 men for the analyses. The stratified analyses based on disease history included 1795 men without history of CHD or congestive heart failure and 655 men with history of these diseases. For the dietary analyses we excluded men with missing data on dietary intakes (n = 72), leaving 2610 men for the analyses, 1902 men without history of CHD or congestive heart failure and 708 men with history of these diseases.
Measurements
Subjects gave venous blood samples between 8 A.M. and 10 A.M. at the baseline examinations. The subjects were instructed to abstain from ingesting alcohol for 3 days and from smoking and eating for 12 h before giving the sample. Comprehensive description of the determination of serum lipids and lipoproteins, assessment of medical history and medications, smoking, and alcohol consumption have been reported previously [18].
Physical activity was evaluated based on the 12-month leisure-time physical activity questionnaire and expressed as kcal/day [19]. The most common leisure-time physical activities were recorded, including the average duration, intensity, and frequency of each activity. BMI was computed as the ratio of weight in kilograms to the square of height in meters. Education and annual income were assessed using self-administered questionnaires. Hypertension diagnosis was defined as systolic/diastolic blood pressure > 140/90 mmHg at study visit or use of hypertension medication [18]. Dietary intakes were assessed using 4-day food recording at the time of blood sampling [13]. Nutrient intakes were estimated using the NUTRICA® 2.5 software (Social Insurance Institution, Turku, Finland). The databank of the software is mainly based on Finnish values of nutrient composition of foods. For the total n-6 PUFA intake, we used the sum of LA and AA intakes. The database does not have information on GLA or DGLA content in foods. Healthy Nordic diet score used in the analyses is based on the Baltic Sea diet score [20] but modified slightly to comply with the availability of the dietary data in the KIHD cohort [21]. The score ranges from 0 to 25. The higher the score, the higher the adherence to a healthy Nordic diet.
Serum fatty acid measurements
Serum esterified and nonesterified fatty acids were measured in 1991 from samples that had been stored at − 80 °C in one gas chromatographic run without preseparation, as described previously [22]. Serum fatty acids were extracted with chloroform–methanol. Chloroform phase was evaporated and treated with sodium methoxide, which methylated esterified fatty acids. Quantification was carried out with reference standards (Check Prep Inc., Elysian, MN). Each analyte had individual reference standard, and an internal standard was eicosan. Fatty acids were chromatographed in an NB-351 capillary column (HNU-Nordion, Helsinki, Finland) by a Hewlett-Packard 5890 Series II gas chromatograph (Hewlett-Packard Company, Avondale, PA, since 1999 Agilent Technologies Inc.) with a flame ionization detector. Results were obtained in micromoles per litter and in the data analyses proportion of the fatty acid of the total serum fatty acids was used. The interassay coefficient of variation (CVs) for repeated measurements were 8.7% for LA, 11.6% for GLA, 8.3% for DGLA, and 9.9% for AA. For the serum total n-6 PUFA concentration, we used the sum of LA, GLA, DGLA and AA.
Ascertainment of follow-up events
All AF events that occurred between study entry and December 31, 2014, were included. Data on events were obtained by record linkage from the national computerized hospitalization registry, which covers every hospitalization in Finland. Subjects were hospitalized because of AF or had AF when they were hospitalized for other reasons. Data on vital status were obtained from Statistics Finland. Cardiovascular causes of AF were coded according to International Classification of Diseases codes (8th revision code 427.4, 9th revision code 427.3, and 10th revision code I48) and the accuracy was verified by a physician [23].
Statistical analysis
The univariate associations of the serum n-6 PUFA with demographic, lifestyle and clinical characteristics at baseline were assessed by means and linear regression for continuous variables and Chi2-test for categorical variables. Correlations between the individual n-6 PUFAs were evaluated by Spearman correlation. Cox proportional hazards regression models adjusted for relevant covariates were used to estimate hazard ratios (HRs) of incident events. The validity of the proportional hazard assumption was evaluated using Schoenfeld residuals, and the assumptions were met. The analyses were controlled for possible confounders, which were selected based on established risk factors for AF [15], or on associations with exposures or outcomes in the present analysis.
Two different models were used to control for confounding factors. The model 1 was adjusted for age (years) and examination year. The multivariable model 2 included model 1 and BMI (kg/m2), smoking (pack/years), years of education, leisure-time physical activity (kilocalories/day), intake of alcohol (grams/week), serum triglycerides (mmol/L), serum long-chain n-3 PUFA concentration (% of all serum fatty acids), systolic and diastolic blood pressure (mm Hg), family history of ischemic heart disease, and use of hypercholesterolemia or hypertension medications at baseline or during follow-up (yes or no). In the analyses with dietary n-6 PUFA intakes, also total energy intake was used as a covariate. Additional adjustments for serum HDL cholesterol or LDL cholesterol concentrations, income, history of type 2 diabetes, or intakes of fruits, berries and vegetables, eggs, whole grains, meat, fish, dairy, butter, or a healthy Nordic diet score did not appreciably change the associations (< 5% change in estimates).”
Cohort means were used to replace missing values in covariates (< 0.5%). Statistical significance of the interactions on a multiplicative scale was assessed by likelihood ratio tests with a cross-product term. Tests of linear trend across categories were conducted by assigning the median values for each category of exposure variable and treating those as a single continuous variable. Potential nonlinear associations were assessed semi-parametrically using restricted cubic splines. All P values were two-sided (α = 0.05). Data were analyzed using the SPSS software version 27 for windows (Armonk, NY: IBM Corp.) and Stata version 14.1 for spline analysis (Stata Corp).
Results
Baseline characteristics
The mean (SD) age of the participants was 53.0 (5.2) years. The mean (SD) serum concentrations, as a percentage of all serum fatty acids, were 32.79% (4.85) for serum total n-6 PUFA concentration, 26.40% (4.65) for LA, 0.29% (0.11) for GLA, 1.34% (0.30) for DGLA, and 4.77% (1.01) for AA. The intercorrelations between the individual n-6 PUFA were weak, except for a moderate correlation between GLA and DGLA; (−0.19 for LA and GLA, P < 0.001), (− 0.09 for LA and DGLA, P < 0.001), (0.13 for LA and AA, P < 0.001), (0.54 for GLA and DGLA, P < 0.001), (0.19 for GLA and AA, P < 0.001), (0.09 for DGLA and AA, P < 0.001).
Baseline characteristics of the participants according to quartiles of the total n-6 PUFA concentration are presented in the Table 1. Men with higher concentration were more likely to have a higher annual income and education, leisure-time physical activity, and serum HDL and LDL cholesterol concentrations, higher intakes of LA and AA, fruits, berries and vegetables, eggs, total grains, whole grains, red meat and total meat products, and a higher adherence to a healthy Nordic diet, but lower serum triglyceride concentration and systolic and diastolic blood pressure, and lower intakes of fish, dairy products, and butter. They also had a lower BMI and alcohol intake and were less likely to have hypertension, congestive heart failure, ischemic heart disease, diabetes, and to smoke.
Associations between serum the n-6 PUFA and risk of AF
During the mean follow-up of 22.4 years, AF was diagnosed in 486 (19.8%) of the 2450 men. The risk for AF was 33% lower (HR 0.76, 95% CI 0.59–0.99, P trend across quartiles = 0.007) in the highest vs. the lowest serum total n-6 PUFA quartile after adjustment for age and examination year (Model 1, Table 2). Further adjustments for potential confounders slightly attenuated the association (extreme-quartile HR 0.79, 95% CI 0.58–1.08, P trend = 0.04) (Model 2, Table 2). Among the individual fatty acids, only LA was associated with reduced risk of AF (the multivariable-adjusted extreme-quartile HR 0.69, 95% CI 0.51–0.94, P trend = 0.02) (Model 2, Table 2). GLA, DGLA and AA were not associated with the risk of AF (Table 2). Similarly, when evaluated continuously, 1-SD increase in serum total n-6 PUFA and serum LA concentrations were associated with 15% lower risk of AF (HR 0.85, 95% CI 0.75–0.95 and HR 0.85, 95% CI 0.76–0.96, respectively) (Fig. 2). Restricted cubic splines analysis confirmed a relatively linear inverse association of serum total n-6 PUFA and LA with the risk of AF and showed little evidence for nonlinearity (Fig. 1). No associations were observed between GLA, DGLA, AA and risk of AF (Table 2, Supplemental Fig. 1).
Multivariable-adjusted hazard ratios of serum n–6 PUFAs and serum LA with risk of atrial fibrillation among 2450 men, evaluated by restricted cubic splines from Cox proportional hazards models. The models were adjusted for age (years), examination year, body mass index (kg/m2), smoking (pack/years), years of education, leisure-time physical activity (kilocalories/day), intake of alcohol (grams/week), serum triglycerides (mmol/L), serum long-chain n-3 PUFA concentration (%), systolic and diastolic blood pressures (mm Hg), family history of ischemic heart disease, and use of hypercholesterolemia or hypertension medications at baseline or during follow-up (yes or no). The solid lines represent the central risk estimates, and the shaded areas represent 95% CIs, relative to the reference level (12.5th percentile). The dotted vertical lines correspond to the 10th, 25th, 50th, 75th and 90th percentiles of fatty acid concentrations. Abbreviations: LA, linoleic acid; PUFA, polyunsaturated fatty acids
Sensitivity analyses
The associations of the total n-6 PUFA and LA were slightly stronger among the subjects without history of CHD or congestive heart failure at baseline, whereas no statistically significant associations were observed among the men with history of these diseases (P-interaction = 0.05, Fig. 2). Among the 1795 men without disease history, 319 AF events occurred. Each 1-SD increase in serum total n-6 PUFA and LA concentrations were associated with a multivariable-adjusted 22% (95% CI 8–34%) and 21% (95% CI 7–33%) lower risk of AF, respectively (Fig. 2). There were no statistically significant associations between the serum GLA, DGLA and AA and risk of AF in these analyses, either (P for interactions ≥ 0.61) (Fig. 2).
Multivariable-adjusted hazard ratios of atrial fibrillation per 1 SD increase in the serum fatty acid concentrations. The models were adjusted for age (years), examination year, body mass index (kg/m2), smoking (pack/years), years of education, leisure-time physical activity (kilocalories/day), intake of alcohol (grams/week), serum triglycerides (mmol/L), serum long-chain n-3 PUFA concentration (%), systolic and diastolic blood pressures (mm Hg), family history of ischemic heart disease, and use of hypercholesterolemia or hypertension medications at baseline or during follow-up (yes or no). Abbreviations: AA, arachidonic acid; DGLA, dihomo-γ-linolenic acid; GLA, γ-linolenic acid; LA, linoleic acid; PUFA, polyunsaturated fatty acids
Associations between dietary n-6 PUFA intakes and risk of AF
During the follow-up, among the 1902 men without disease history, 335 AF events occurred, while AF was diagnosed in 176 of the 708 men with disease history. Similar associations with the risk of AF as with the serum n-6 PUFA concentrations were also observed with the intakes of total n-6 PUFA, LA and AA. Higher intakes of total n-6 PUFA and LA were associated with reduced risk of AF [the multivariable-adjusted extreme-quartile HR = 0.66 (95% CI 0.43–0.98, P trend = 0.04) for total n-6 PUFA, and HR = 0.64 (95% CI 0.43–0.95, P trend = 0.03)] for LA, only among those without history of CHD or congestive heart failure at baseline (Supplemental Table 1). There were no statistically significant associations between the dietary intake of AA and risk of AF among men with or without disease history (Supplemental Table 1).
Discussion
In this population-based cohort study among middle-aged and older men from eastern Finland, higher serum concentration and dietary intake of LA, the major n-6 PUFA, were associated with lower risk of AF and the associations were evident especially among those without history of CHD and congestive heart failure. Serum GLA, DGLA, and AA were not associated with the risk.
Only a few studies have evaluated the associations of the n-6 PUFA with the risk of AF, with heterogeneous findings. In a multi-ethnic cohort of 6229 participants free of CVD (813 AF cases), plasma concentrations of n-6 PUFAs, particularly AA, were associated with lower risk of incident AF [9]. In a Mendelian randomization study among 588,190 individuals (65,446 AF cases), genetically-predicted higher plasma LA concentration, but not AA concentration, had a suggestive inverse association with the risk of AF [10]. This finding with LA is in contrast with the results of the Diet, Cancer and Health cohort study among 57,053 Danish individuals aged 50–65 years (2274 AF cases), where intakes of LA and AA were not associated with the risk of AF [11]. However, in another study from this Danish cohort, adipose tissue concentration of the minor n-6 PUFAs (sum of GLA, DGLA and AA) was associated with a lower risk of developing AF (4710 AF cases) [12]. In contrast, LA was associated with an increased risk of AF in men, but not in women [12].
Although mechanisms underlying the possible antiarrhythmic properties of LA are not well established and future research is needed, some potential factors to explain the inverse association between serum LA and risk of AF could include the favorable impact of LA on the lipoprotein metabolism [24], atrial ischemia [25], and blood pressure [26] that have been shown to be associated with the risk of AF [27,28,29]. Other potential mechanisms include the beneficial properties of LA in the reduction of arterial stiffness and vascular resistance, promotion of endothelium-vasodilation and improvement of pulse wave velocity, which are related to the risk of cardiac arrhythmias, especially among healthy populations [30, 31].
Presence of CHD and congestive heart failure are identified to be independent predictors of AF development [15]. In the present study, the inverse association between serum total n-6 PUFA and LA and risk of AF was observed mainly among those without history of CHD and congestive heart failure. This might be partially explained by the favorable systematic changes in the modifiable risk factors, e.g., diet modification, of AF in participants with the history of cardiac events, which may weaken the association of total n-6 PUFA and LA with the risk of AF. Moreover, this finding suggests that the observed inverse association between serum LA and risk of AF is not mediated by the impact of n-6 PUFAs on the risk of prior cardiac events, specifically CHD and congestive heart failure.
A strength of this study is the use of serum n-6 PUFA in addition to dietary intakes, which also enabled us to investigate the associations with GLA and DGLA. Serum fatty acids are objective biomarkers for exposure and may thus reduce the bias by misclassification that would reduce the associations towards the null in the analyses with dietary intakes. However, analyses with both the serum concentrations and the dietary intakes gave congruent results. Other strengths include the population-based recruitment, extensive examination of potential confounders, and relatively large numbers of incident AF events.
A limitation in this study was the single measurement of fatty acids and assessment of dietary intakes at baseline for all men, which may cause random error and attenuate the associations. However, relatively strong correlations (r ≥ 0.5) for all n-6 PUFAs were previously observed among the subgroup of men with repeated measurements after 4 and 11 years during the follow-up in KIHD [14]. The participants were middle-aged and older men from Eastern Finland, so the findings may not be generalizable to other populations or to women. Also, because of the observational study design, conclusions about causality cannot be drawn.
In conclusion, higher serum LA concentration and higher dietary LA intake were associated with a lower risk of AF, a common cardiac arrhythmia. The association was more evident among the men without history of CHD or congestive heart failure. AA and the minor n–6 PUFAs, GLA and DGLA, had no association with AF risk. More large-scale studies in diverse populations are needed to confirm these findings and explore the underlying mechanisms.
Abbreviations
- AA:
-
Arachidonic acid
- DGLA:
-
Dihomo-γ-linolenic acid
- GLA:
-
γ-Linolenic acid
- LA:
-
Linoleic acid
- KIHD:
-
Kuopio Ischaemic Heart Disease Risk Factor Study
- PUFA:
-
Polyunsaturated fatty acids
References
Andrade J, Khairy P, Dobrev D, Nattel S (2014) The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 114:1453–1468
Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH (2017) Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 120:1501–1517
Mensink RP, Zock PL, Kester AD, Katan MB (2003) Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 77:1146–1155
Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC, Hu FB (2014) Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation 130:1568–1578
Abedi E, Sahari MA (2014) Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci Nutr 2:443–463
Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PR (2003) Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 38:391–398
Marklund M, Wu JH, Imamura F, Del Gobbo LC, Fretts A, De Goede J, Shi P, Tintle N, Wennberg M, Aslibekyan S (2019) Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality: an individual-level pooled analysis of 30 cohort studies. Circulation 139:2422–2436
Li J, Guasch-Ferré M, Li Y, Hu FB (2020) Dietary intake and biomarkers of linoleic acid and mortality: systematic review and meta-analysis of prospective cohort studies. Am J Clin Nutr 112:150–167
Garg PK, Guan W, Nomura S, Weir N, Karger AB, Duprez D, Heckbert SR, Tsai MY (2021) Plasma ω-3 and ω-6 PUFA concentrations and risk of atrial fibrillation: the multi-ethnic study of atherosclerosis. J Nutr 151:1479–1486
Yuan S, Larsson SC (2019) Plasma phospholipid fatty acids and risk of atrial fibrillation: a Mendelian randomization study. Nutrients 11:1651
Mortensen LM, Lundbye-Christensen S, Schmidt EB, Calder PC, Schierup MH, Tjønneland A, Parner ET, Overvad K (2017) Long-chain n-3 and n-6 polyunsaturated fatty acids and risk of atrial fibrillation: results from a Danish cohort study. PLoS ONE 12:e0190262
Dinesen PT, Rix TA, Joensen AM, Dahm CC, Lundbye-Christensen S, Schmidt EB, Overvad K (2018) Patterns of adipose tissue fatty acids and the risk of atrial fibrillation: a case-cohort study. PLoS ONE 13:e0208833
Virtanen JK, Mursu J, Tuomainen T, Voutilainen S (2014) Dietary fatty acids and risk of coronary heart disease in men: the Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler Thromb Vasc Biol 34:2679–2687
Virtanen JK, Wu JH, Voutilainen S, Mursu J, Tuomainen T (2018) Serum n-6 polyunsaturated fatty acids and risk of death: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 107:427–435
Lau DH, Nattel S, Kalman JM, Sanders P (2017) Modifiable risk factors and atrial fibrillation. Circulation 136:583–596
Salonen JT (1988) Is there a continuing need for longitudinal epidemiologic research? The Kuopio Ischaemic Heart Disease Risk Factor Study. Ann Clin Res 20:46–50
Salonen JT, Salonen R, Seppanen K, Rauramaa R, Tuomilehto J (1991) HDL, HDL2, and HDL3 subfractions, and the risk of acute myocardial infarction. A prospective population study in eastern Finnish men. Circulation 84:129–139
Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R (1992) High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 86:803–811
Lakka TA, Venalainen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT (1994) Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction in men. N Engl J Med 330:1549–1554
Kanerva N, Kaartinen NE, Schwab U, Lahti-Koski M, Männistö S (2014) The Baltic Sea Diet Score: a tool for assessing healthy eating in Nordic countries. Public Health Nutr 17:1697–1705
Tertsunen H, Hantunen S, Tuomainen T, Virtanen JK (2020) Healthy Nordic diet and risk of disease death among men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Nutr 59:3545–3553
Laaksonen D, Lakka T, Lakka H, Nyyssönen K, Rissanen T, Niskanen L, Salonen J (2002) Serum fatty acid composition predicts development of impaired fasting glycaemia and diabetes in middle-aged men. Diabetic Med 19:456–464
Virtanen J, Mursu J, Voutilainen S, Tuomainen T (2010) Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Altern Med Rev 15:89–90
Tourtas T, Birke MT, Kruse FE, Welge-Lüssen U, Birke K (2012) Preventive effects of omega-3 and omega-6 Fatty acids on peroxide mediated oxidative stress responses in primary human trabecular meshwork cells. PLoS ONE 7:e31340
Venø SK, Bork CS, Jakobsen MU, Lundbye-Christensen S, Bach FW, Overvad K, Schmidt EB (2018) Linoleic acid in adipose tissue and development of ischemic stroke: a Danish Case-cohort study. J Am Heart Assoc 7:e009820
Miura K, Stamler J, Nakagawa H, Elliott P, Ueshima H, Chan Q, Brown IJ, Tzoulaki I, Saitoh S, Dyer AR (2008) Relationship of dietary linoleic acid to blood pressure: the international study of macro-micronutrients and blood pressure study. Hypertension 52:408–414
Thomas M, Dublin S, Kaplan RC, Glazer N, Lumley T, Longstreth W, Smith N, Psaty B, Siscovick D, Heckbert S (2008) Blood pressure control and risk of incident atrial fibrillation. Am J Hypertens 21:1111–1116
Richter B, Gwechenberger M, Socas A, Zorn G, Albinni S, Marx M, Bergler-Klein J, Binder T, Wojta J, Gössinger HD (2012) Markers of oxidative stress after ablation of atrial fibrillation are associated with inflammation, delivered radiofrequency energy and early recurrence of atrial fibrillation. Clin Res Cardiol 101:217–225
Iwasaki Y, Nishida K, Kato T, Nattel S (2011) Atrial fibrillation pathophysiology: implications for management. Circulation 124:2264–2274
Sarabi M, Vessby B, Millgård J, Lind L (2001) Endothelium-dependent vasodilation is related to the fatty acid composition of serum lipids in healthy subjects. Atherosclerosis 156:349–355
West SG, Krick AL, Klein LC, Zhao G, Wojtowicz TF, McGuiness M, Bagshaw DM, Wagner P, Ceballos RM, Holub BJ (2010) Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. J Am Coll Nutr 29:595–603
Acknowledgements
The present study was supported by the University of Eastern Finland. The KIHD project was mainly funded by research grants from the NIH and the Finnish Academy to Jukka T. Salonen and George A. Kaplan. The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital.
Author information
Authors and Affiliations
Contributions
BT, T-PT, JTS and JKV acquired the data and designed and conducted the research. BT analyzed the data and drafted the manuscript. T-PT, MI, JTS and JKV critically revised the manuscript for important intellectual content; and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
394_2021_2780_MOESM1_ESM.jpg
Supplementary figure 1. (JPG 324 KB). Multivariable-adjusted hazard ratios of serum AA, DGLA, and GLA with risk of atrial fibrillation among 2450 men, evaluated by restricted cubic splines from Cox proportional hazards models. The models were adjusted for age (years), examination year, body mass index (kg/m2), smoking (pack/years), years of education, leisure-time physical activity (kilocalories/day), intake of alcohol (grams/week), serum triglycerides (mmol/L), serum long-chain n-3 PUFA concentration (%), systolic and diastolic blood pressures (mm Hg), family history of ischemic heart disease, and use of hypercholesterolemia or hypertension medications at baseline or during follow-up (yes or no). The solid lines represent the central risk estimates, and the shaded areas represent 95% CIs, relative to the reference level (12.5th percentile). The dotted vertical lines correspond to the 10th, 25th, 50th, 75th and 90th percentiles of fatty acid concentrations. Abbreviations: AA, arachidonic acid; DGLA, dihomo-γ-linolenic acid; GLA, γ -linolenic acid.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tajik, B., Tuomainen, TP., Isanejad, M. et al. Serum n-6 polyunsaturated fatty acids and risk of atrial fibrillation: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Nutr 61, 1981–1989 (2022). https://doi.org/10.1007/s00394-021-02780-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02780-0