Abstract
Purpose
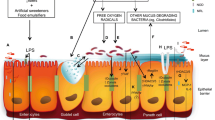
Aging can be characterized by increased systemic low-grade inflammation, altered gut microbiota composition, and increased intestinal permeability (IP). The intake of polyphenol-rich foods is proposed as a promising strategy to positively affect the gut microbiota–immune system–intestinal barrier (IB) axis. In this context, we tested the hypothesis that a PR-dietary intervention would affect the presence of bacterial factors in the bloodstream of older adults.
Methods
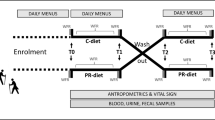
We collected blood samples within a randomized, controlled, crossover intervention trial in which older volunteers (n = 51) received a polyphenol-enriched and a control diet. We quantified the presence of bacterial DNA in blood by qPCR targeting the 16S rRNA gene (16S; bacterial DNAemia). Blood DNA was taxonomically profiled via 16S sequencing.
Results
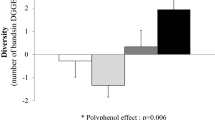
Higher blood 16S levels were associated with higher BMI and markers of IP, inflammation, and dyslipidemia. PR-intervention did not significantly change bacterial DNAemia in the older population (P = 0.103). Nonetheless, the beneficial changes caused by the polyphenol-enriched diet were greatest in participants with higher bacterial DNAemia, specifically in markers related to IP, inflammation and dyslipidemia, and in fecal bacterial taxa. Finally, we found that the bacterial DNA detected in blood mostly belonged to γ-Proteobacteria, whose abundance significantly decreased after the polyphenol-rich diet in subjects with higher bacterial DNAemia at baseline.
Conclusions
This study shows that older subjects with higher bacterial DNAemia experienced a beneficial effect from a polyphenol-rich diet. Bacterial DNAemia may be a further relevant marker for the identification of target populations that could benefit more from a protective dietary treatment.
Registration
This trial was retrospectively registered at www.isrctn.org (ISRCTN10214981) on April 28, 2017.



Similar content being viewed by others
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request in a deidentified form pending review and approval of a formal request to the scientific coordinators of the MaPLE project (patrizia.riso@unimi.it; simone.guglielmetti@unimi.it; candres@ub.edu; paul.kroon@quadram.ac.uk).
References
Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69(Suppl 1):S4-9. https://doi.org/10.1093/gerona/glu057
Bernardi S, Del Bo C, Marino M, Gargari G, Cherubini A, Andres-Lacueva C, Hidalgo-Liberona N, Peron G, Gonzalez-Dominguez R, Kroon P, Kirkup B, Porrini M, Guglielmetti S, Riso P (2020) Polyphenols and intestinal permeability: rationale and future perspectives. J Agric Food Chem 68(7):1816–1829. https://doi.org/10.1021/acs.jafc.9b02283
Cox AJ, West NP, Cripps AW (2015) Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol 3(3):207–215. https://doi.org/10.1016/S2213-8587(14)70134-2
Guerreiro CS, Calado A, Sousa J, Fonseca JE (2018) Diet, microbiota, and gut permeability-the unknown triad in rheumatoid arthritis. Front Med 5:349. https://doi.org/10.3389/fmed.2018.00349
Brandl K, Schnabl B (2017) Intestinal microbiota and nonalcoholic steatohepatitis. Curr Opin Gastroenterol 33(3):128–133. https://doi.org/10.1097/MOG.0000000000000349
Forkosh E, Ilan Y (2019) The heart-gut axis: new target for atherosclerosis and congestive heart failure therapy. Open heart 6(1):e000993. https://doi.org/10.1136/openhrt-2018-000993
Michielan A, D’Inca R (2015) Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediat Inflamm 2015:628157. https://doi.org/10.1155/2015/628157
Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP (2015) Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9:392. https://doi.org/10.3389/fncel.2015.00392
Natividad JM, Verdu EF (2013) Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res 69(1):42–51. https://doi.org/10.1016/j.phrs.2012.10.007
Sovran B, Hugenholtz F, Elderman M, Van Beek AA, Graversen K, Huijskes M, Boekschoten MV, Savelkoul HFJ, De Vos P, Dekker J, Wells JM (2019) Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci Rep 9(1):1437. https://doi.org/10.1038/s41598-018-35228-3
Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, Schertzer JD, Larche MJ, Davidson DJ, Verdu EF, Surette MG, Bowdish DME (2017) Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21(4):455-466e454. https://doi.org/10.1016/j.chom.2017.03.002
Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S (2020) Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutr 11(1):77–91. https://doi.org/10.1093/advances/nmz061
Hu J, Luo H, Wang J, Tang W, Lu J, Wu S, Xiong Z, Yang G, Chen Z, Lan T, Zhou H, Nie J, Jiang Y, Chen P (2017) Enteric dysbiosis-linked gut barrier disruption triggers early renal injury induced by chronic high salt feeding in mice. Exp Mol Med 49(8):e370. https://doi.org/10.1038/emm.2017.122
De Santis S, Cavalcanti E, Mastronardi M, Jirillo E, Chieppa M (2015) Nutritional keys for intestinal barrier modulation. Front Immunol 6:612. https://doi.org/10.3389/fimmu.2015.00612
Peron G, Hidalgo-Liberona N, Gonzalez-Dominguez R, Garcia-Aloy M, Guglielmetti S, Bernardi S, Kirkup B, Kroon PA, Cherubini A, Riso P, Andres-Lacueva C (2020) Exploring the molecular pathways behind the effects of nutrients and dietary polyphenols on gut microbiota and intestinal permeability: a perspective on the potential of metabolomics and future clinical applications. J Agric Food Chem 68(7):1780–1789. https://doi.org/10.1021/acs.jafc.9b01687
Roman B, Carta L, Martinez-Gonzalez MA, Serra-Majem L (2008) Effectiveness of the Mediterranean diet in the elderly. Clin Interv Aging 3(1):97–109. https://doi.org/10.2147/cia.s1349
Correa RCG, Peralta RM, Haminiuk CWI, Maciel GM, Bracht A, Ferreira I (2018) New phytochemicals as potential human anti-aging compounds: reality, promise, and challenges. Crit Rev Food Sci Nutr 58(6):942–957. https://doi.org/10.1080/10408398.2016.1233860
Guglielmetti S, Bernardi S, Del Bo C, Cherubini A, Porrini M, Gargari G, Hidalgo-Liberona N, Gonzalez-Dominguez R, Peron G, Zamora-Ros R, Winterbone MS, Kirkup B, Kroon PA, Andres-Lacueva C, Riso P (2020) Effect of a polyphenol-rich dietary pattern on intestinal permeability and gut and blood microbiomics in older subjects: study protocol of the MaPLE randomised controlled trial. BMC Geriatr 20(1):77. https://doi.org/10.1186/s12877-020-1472-9
Del Bo C, Bernardi S, Cherubini A, Porrini M, Gargari G, Hidalgo-Liberona N, Gonzalez-Dominguez R, Zamora-Ros R, Peron G, Marino M, Gigliotti L, Winterbone MS, Kirkup B, Kroon PA, Andres-Lacueva C, Guglielmetti S, Riso P (2021) A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: the MaPLE randomised controlled trial. Clin Nutr 40(5):3006–3018. https://doi.org/10.1016/j.clnu.2020.12.014
Fasano A (2012) Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 10(10):1096–1100. https://doi.org/10.1016/j.cgh.2012.08.012
Martini D, Bernardi S, Del Bo C, Hidalgo Liberona N, Zamora-Ros R, Tucci M, Cherubini A, Porrini M, Gargari G, Gonzalez-Dominguez R, Peron G, Kirkup B, Kroon PA, Andres-Lacueva C, Guglielmetti S, Riso P (2020) Estimated intakes of nutrients and polyphenols in participants completing the MaPLE randomised controlled trial and its relevance for the future development of dietary guidelines for the older subjects. Nutrients. https://doi.org/10.3390/nu12082458
Paisse S, Valle C, Servant F, Courtney M, Burcelin R, Amar J, Lelouvier B (2016) Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 56(5):1138–1147. https://doi.org/10.1111/trf.13477
Nadkarni MA, Martin FE, Jacques NA, Hunter N (2002) Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148(Pt 1):257–266. https://doi.org/10.1099/00221287-148-1-257
Lluch J, Servant F, Paisse S, Valle C, Valiere S, Kuchly C, Vilchez G, Donnadieu C, Courtney M, Burcelin R, Amar J, Bouchez O, Lelouvier B (2015) The characterization of novel tissue microbiota using an optimized 16S metagenomic sequencing pipeline. PLoS ONE 10(11):e0142334. https://doi.org/10.1371/journal.pone.0142334
Gargari G, Mantegazza G, Taverniti V, Del Bo C, Bernardi S, Andres-Lacueva C, Gonzalez-Dominguez R, Kroon PA, Winterbone MS, Cherubini A, Riso P, Guglielmetti S (2021) Bacterial DNAemia is associated with serum zonulin levels in older subjects. Sci Rep 11(1):11054. https://doi.org/10.1038/s41598-021-90476-0
Antuna-Puente B, Disse E, Rabasa-Lhoret R, Laville M, Capeau J, Bastard JP (2011) How can we measure insulin sensitivity/resistance? Diabetes Metab 37(3):179–188. https://doi.org/10.1016/j.diabet.2011.01.002
Curtis JR, John A, Baser O (2012) Dyslipidemia and changes in lipid profiles associated with rheumatoid arthritis and initiation of anti-tumor necrosis factor therapy. Arthritis Care Res 64(9):1282–1291. https://doi.org/10.1002/acr.21693
Wang Z, Huang W, Li H, Tang L, Sun H, Liu Q, Zhang L (2018) Synergistic action of inflammation and lipid dysmetabolism on kidney damage in rats. Ren Fail 40(1):175–182. https://doi.org/10.1080/0886022X.2018.1450763
Schumacher SM, Naga Prasad SV (2018) Tumor necrosis factor-alpha in heart failure: an updated review. Curr Cardiol Rep 20(11):117. https://doi.org/10.1007/s11886-018-1067-7
Ling Z, Liu X, Cheng Y, Shao L, Jiang H, Li L (2017) Blood microbiota as a potential noninvasive diagnostic biomarker for liver fibrosis in severely obese patients: choose carefully. Hepatology 65(5):1775–1776. https://doi.org/10.1002/hep.28987
Shah NB, Allegretti AS, Nigwekar SU, Kalim S, Zhao S, Lelouvier B, Servant F, Serena G, Thadhani RI, Raj DS, Fasano A (2019) Blood microbiome profile in CKD: a pilot study. Clin J Am Soc Nephrol CJASN 14(5):692–701. https://doi.org/10.2215/CJN.12161018
Anhê FF, Jensen BAH, Varin TV, Servant F, Van Blerk S, Richard D, Marceau S, Surette M, Biertho L, Lelouvier B, Schertzer JD, Tchernof A, Marette A (2020) Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat Metab 2(3):233–242. https://doi.org/10.1038/s42255-020-0178-9
Amar J, Lelouvier B, Servant F, Lluch J, Burcelin R, Bongard V, Elbaz M (2019) Blood microbiota modification after myocardial infarction depends upon low-density lipoprotein cholesterol levels. J Am Heart Assoc 8(19):e011797. https://doi.org/10.1161/JAHA.118.011797
Tian M, Yuan YC, Li JY, Gionfriddo MR, Huang RC (2015) Tumor necrosis factor-alpha and its role as a mediator in myocardial infarction: a brief review. Chronic Dis Transl Med 1(1):18–26. https://doi.org/10.1016/j.cdtm.2015.02.002
Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernandez-Real JM (2012) Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS ONE 7(5):e37160. https://doi.org/10.1371/journal.pone.0037160
Kim AS, Ko HJ (2018) Plasma concentrations of zonulin are elevated in obese men with fatty liver disease. Diabetes Metab Syndr Obes Targets Ther 11:149–157. https://doi.org/10.2147/DMSO.S163062
Seekatz AM, Schnizlein MK, Koenigsknecht MJ, Baker JR, Hasler WL, Bleske BE, Young VB, Sun D (2019) Spatial and temporal analysis of the stomach and small-intestinal microbiota in fasted healthy humans. mSphere. https://doi.org/10.1128/mSphere.00126-19
Hidalgo-Liberona N, Gonzalez-Dominguez R, Vegas E, Riso P, Del Bo C, Bernardi S, Peron G, Guglielmetti S, Gargari G, Kroon PA, Cherubini A, Andres-Lacueva C (2020) Increased intestinal permeability in older subjects impacts the beneficial effects of dietary polyphenols by modulating their bioavailability. J Agric Food Chem 68(44):12476–12484. https://doi.org/10.1021/acs.jafc.0c04976
Scheffler L, Crane A, Heyne H, Tonjes A, Schleinitz D, Ihling CH, Stumvoll M, Freire R, Fiorentino M, Fasano A, Kovacs P, Heiker JT (2018) Widely used commercial ELISA does not detect precursor of Haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front Endocrinol 9:22. https://doi.org/10.3389/fendo.2018.00022
Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, Arrietta MC, Meddings JB, Fasano A (2009) Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA 106(39):16799–16804. https://doi.org/10.1073/pnas.0906773106
Oliviero S, Cortese R (1989) The human haptoglobin gene promoter: interleukin-6-responsive elements interact with a DNA-binding protein induced by interleukin-6. EMBO J 8(4):1145–1151
Brock M, Trenkmann M, Gay RE, Gay S, Speich R, Huber LC (2011) MicroRNA-18a enhances the interleukin-6-mediated production of the acute-phase proteins fibrinogen and haptoglobin in human hepatocytes. J Biol Chem 286(46):40142–40150. https://doi.org/10.1074/jbc.M111.251793
Qi Y, Goel R, Kim S, Richards EM, Carter CS, Pepine CJ, Raizada MK, Buford TW (2017) Intestinal permeability biomarker zonulin is elevated in healthy aging. J Am Med Dir Assoc 18(9):810e811-810e814. https://doi.org/10.1016/j.jamda.2017.05.018
Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, Mohan V, Balasubramanyam M (2014) Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem 388(1–2):203–210. https://doi.org/10.1007/s11010-013-1911-4
Fasano A (2017) Gut permeability, obesity, and metabolic disorders: who is the chicken and who is the egg? Am J Clin Nutr 105(1):3–4. https://doi.org/10.3945/ajcn.116.148338
Vassalle C, Mazzone A, Sabatino L, Carpeggiani C (2016) Uric Acid for Cardiovascular Risk: Dr. Jekyll or Mr. Hide? Diseases. https://doi.org/10.3390/diseases4010012
Yamakoshi J, Tokutake S, Kikuchi M, Kubota Y, Konishi H, Mitsuoka T (2001) Effect of proanthocyanidin-rich extract from grape seeds on human fecal flora and fecal odor. Microb Ecol Health Dis 13(1):25–31. https://doi.org/10.1080/089106001750071672
Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP (2011) Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr 93(1):62–72. https://doi.org/10.3945/ajcn.110.000075
Guglielmetti S, Fracassetti D, Taverniti V, Del Bo’ C, Vendrame S, Klimis-Zacas D, Arioli S, Riso P, Porrini M (2013) Differential modulation of human intestinal bifidobacterium populations after consumption of a wild blueberry (Vaccinium angustifolium) drink. J Agric Food Chem 61(34):8134–8140. https://doi.org/10.1021/jf402495k
Vendrame S, Guglielmetti S, Riso P, Arioli S, Klimis-Zacas D, Porrini M (2011) Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J Agric Food Chem 59(24):12815–12820. https://doi.org/10.1021/jf2028686
Gwiazdowska D, Juś K, Jasnowska-Małecka J, Kluczyńska K (2015) The impact of polyphenols on Bifidobacterium growth. Acta Biochim Pol 62(4):895–901. https://doi.org/10.18388/abp.2015_1154
Tabasco R, Sánchez-Patán F, Monagas M, Bartolomé B, Victoria Moreno-Arribas M, Peláez C, Requena T (2011) Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: resistance and metabolism. Food Microbiol 28(7):1345–1352. https://doi.org/10.1016/j.fm.2011.06.005
Schierwagen R, Alvarez-Silva C, Madsen MSA, Kolbe CC, Meyer C, Thomas D, Uschner FE, Magdaleno F, Jansen C, Pohlmann A, Praktiknjo M, Hischebeth GT, Molitor E, Latz E, Lelouvier B, Trebicka J, Arumugam M (2018) Circulating microbiome in blood of different circulatory compartments. Gut. https://doi.org/10.1136/gutjnl-2018-316227
Rajendhran J, Shankar M, Dinakaran V, Rathinavel A, Gunasekaran P (2013) Contrasting circulating microbiome in cardiovascular disease patients and healthy individuals. Int J Cardiol 168(5):5118–5120. https://doi.org/10.1016/j.ijcard.2013.07.232
Li Q, Wang C, Tang C, Zhao X, He Q, Li J (2018) Identification and characterization of blood and neutrophil-associated microbiomes in patients with severe acute pancreatitis using next-generation sequencing. Front Cell Infect Microbiol 8:5. https://doi.org/10.3389/fcimb.2018.00005
Nikkari S, McLaughlin IJ, Bi W, Dodge DE, Relman DA (2001) Does blood of healthy subjects contain bacterial ribosomal DNA? J Clin Microbiol 39(5):1956–1959. https://doi.org/10.1128/JCM.39.5.1956-1959.2001
Vaure C, Liu Y (2014) A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol 5:316. https://doi.org/10.3389/fimmu.2014.00316
Acknowledgements
We sincerely thank all the volunteers for their valuable contribution and dedication to the study. We are grateful to the nursing and medical staff working at Opera Immacolata Concezione (OIC Foundation, Padua, Italy) for their coordinating activities in the nursing home and for the success of the study.
Funding
This work was completed as part of the MAPLE project (Gut and Blood Microbiomics for Studying the Effect of a Polyphenol-Rich Dietary Pattern on Intestinal Permeability in the Elderly) supported within the European Joint Programming Initiative “A Healthy Diet for a Healthy Life” (JPI-HDHL, http://www.healthydietforhealthylife.eu/) granted by Mipaaft (Italy, D.M. 8245/7303/ 2016), MINECO (Spain, PCIN-2015-238), and BBSRC (U.K., BB/R012512/1). This work also received funding from the JPI-HDHL ERA-Net Cofund on INtesTInal MICrobiomics (ERA-HDHL INTIMIC, AC19/00096), CIBERFES funded by Instituto de Salud Carlos III and co-funded by the European Regional Development Fund "A way to make Europe"; 2017SGR1546 grant from the Generalitat de Catalunya’s Agency AGAUR, and ICREA Academia Award 2018. Additional funding was provided by the Biotechnology and Biological Sciences Research Council (UK) through an Institute Strategic Programme Grant (‘Food Innovation and Health’; Grant No. BB/R012512/1 1 and its constituent projects BBS/E/F/000PR10343 (Theme 1, Food Innovation) and BBS/E/F/000PR10346 (Theme 3, Digestion and Fermentation in the Lower GI Tract) to the Quadram Institute Bioscience. TM thanks the “Juan de la Cierva” program from MINECO (IJCI-2017-32534).
Author information
Authors and Affiliations
Contributions
SG, Guarantor of article. PR and SG designed the trial and in collaboration with AC, CAL, and PAK optimized the study protocol including the development of the polyphenol-rich diet. SG drafted the first version of the manuscript in collaboration with GG, CDB, and PR. Laboratory work was carried out by VT, GG, NHL, and SB. GG performed bioinformatic analyses in collaboration with SG. GG and SG performed the statistical analysis in collaboration with TM. All the authors critically revised the draft and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors declares conflict of interest.
Ethical approval
The study protocol was approved by the Ethics Committee of the University of Milan (ref: 6/16/CE_15.02.16_Verbale_All-7). All participants were informed about the study protocol and signed an informed consent before the enrolment.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gargari, G., Taverniti, V., Del Bo’, C. et al. Higher bacterial DNAemia can affect the impact of a polyphenol-rich dietary pattern on biomarkers of intestinal permeability and cardiovascular risk in older subjects. Eur J Nutr 61, 1209–1220 (2022). https://doi.org/10.1007/s00394-021-02680-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02680-3