Abstract
Purpose
Reducing postprandial hyperglycemia has beneficial effects on diabetes-related risk factors, but the magnitude of the reduction needed to achieve such an effect is unknown. The purpose of the study was to quantify the relationship of acute glucose and insulin postprandial responses with longer-term effects on diabetes-related risk factors by performing a systematic review and meta-analysis of dietary intervention studies.
Methods
We systematically searched EMBASE and MEDLINE. Dietary intervention studies among any human population aiming to reduce postprandial glycemia, with actual measures of postprandial glucose (PPG) and/or insulin (PPI) as acute exposures (incremental area under the curve, iAUC) as well as markers of glucose metabolism (fasting glucose, HbA1c) and insulin sensitivity (fasting insulin, HOMA-IR) after at least 4 weeks of diet intervention as outcomes were included. Meta-analyses were performed for the effects on acute exposures and on diabetes-related risk factors. The relationship between changes in acute exposures and changes in risk factor outcomes was estimated by meta-regression analyses.
Results
Out of the 13,004 screened papers, 13 papers with 14 comparisons were included in the quantitative analysis. The dietary interventions acutely reduced mean PPG [mean difference (MD), − 0.27 mmol/l; 95% CI − 0.41 to − 0.14], but not mean PPI (MD − 7.47 pmol/l; 95% CI − 16.79 to 1.86). There were no significant overall effects on fasting glucose and insulin. HbA1c was reduced by − 0.20% (95% CI − 0.35 to − 0.05). Changes in acute PPG were significantly associated with changes in fasting plasma glucose (FPG) [per 10% change in PPG: β = 0.085 (95% CI 0.003, 0.167), k = 14], but not with fasting insulin [β = 1.20 (95% CI − 0.32, 2.71), k = 12]. Changes in acute PPI were not associated with changes in FPG [per 10% change in PPI: β = − 0.017 (95% CI − 0.056, 0.022), k = 11].
Conclusions
Only a limited number of postprandial glucose-lowering dietary intervention studies measured acute postprandial exposures to PPG/PPI during the interventions. In this small heterogeneous set of studies, an association was found between the magnitude of the acute postprandial responses and the change in fasting glucose, but no other outcomes. More studies are needed to quantify the relationship between acute postprandial changes and long-term effects on risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and type 2 diabetes (T2D) are major global concerns. Recent estimates of T2D expect dramatic increases by 2035 to reach 471 million of cases globally [1]. Postprandial hyperglycemia, as well as the related phenomena of hyperinsulinemia and hyperlipemia, has been implicated in the etiology of chronic metabolic diseases such as T2D [1]. Moreover, elevated fasting and postprandial glucose levels are consistently associated with an increased risk of cardiovascular events, even in the non-diabetic range [3]. To prevent diabetes, an integrated approach is required which includes both dietary modification and regular physical activity [4,5,6]. Indeed, in non-diabetic hyperglycemia, lifestyle treatment or medication to improve glycemic control was associated with a reduced risk of future diabetes [7].
A number of papers have hypothesized the value of consuming low glycemic index foods to decrease the overall glycemic response of the diet for long-term benefit. Meta-analyses of the effect of low glycemic index (GI) diets indeed demonstrated beneficial effects on body weight in people with obesity and prevention of T2D and cardiovascular diseases [8,9,10].
However, the magnitude of the reduction of postprandial glycemic response using dietary interventions such as low GI foods or meals, compared to high GI interventions in relation to longer-term established diabetes-related risk factors has not been quantified. At the moment, the majority of dietary studies investigate individual foods and their ability to reduce glucose levels over a period of a single meal only. It is therefore important to understand the relevance of these single meal studies by investigating the quantitative reductions in PPG/PPI needed acutely to induce relevant changes on established longer-term risk factors chronically, and disease prevention ultimately. Therefore, the aim of this work was to quantify the relationship between acute glucose and insulin postprandial responses and their effects on diabetes-related risk factors over time by performing a systematic review and meta-analysis of controlled postprandial glucose-lowering dietary intervention studies.
Methods
Data source and searches
The bibliographic databases Elsevier Medical Database (EMBASE) and the US National Library of Medicine database (MEDLINE via the PubMed portal) were systematically searched for relevant papers until September 13, 2019. Relevant papers that were identified while developing the search string or based on authors’ own files were manually included when needed. Search terms were defined by the research question, including terms for GI/glycemic load (GL) dietary interventions, postprandial responses, and study design. Indexed terms were used from MeSH in PubMed and from EMtree in EMBASE. Free-text terms were used in both databases as well. The full search strategies for both databases can be found in Supplementary File 1. The protocol and search strategies used were registered at PROSPERO prior to the study being executed (CRD42018093153).
Study selection
Titles and abstracts were screened in duplicate, independently by pairs of reviewers (MA, JWB, JMD, LE, CR, FS, SV, MDR) and differences were resolved by consensus. Full-text papers were screened independently by two reviewers (MA, JWB, LE, MDR, CR) for eligibility. Studies were included if they: (1) studied any human population, including healthy individuals and individuals with prediabetes, type 1 and type 2 diabetes mellitus; (2) involved any dietary intervention that aimed at reducing GI, GL, or postprandial glucose responses; (3) reported measures of postprandial glucose (PPG) or postprandial insulin (PPI) as acute exposures to study diets; (4) reported measures of glycemic control and/or insulin sensitivity over time as outcomes. Studies were excluded if they: (1) had a study duration < 4 weeks; (2) were not written in the English language; (3) had no control group; (4) had co-interventions; (5) had changes in glucose-lowering medication use during study; (6) had no accessible full text. If eligible full-text papers did not report acute PPG and PPI response data, papers were checked for references to related papers that had previously published this data. Multiple arms of the same study were included when these arms were independent (had different control groups) [11].
Data extraction
Data extraction of the included studies was performed by one reviewer (CR) and was appraised (for a random subsample) by a second reviewer (MA). Information on study design, population, intervention diet, acute PPG and PPI exposures (levels per time point, AUC, incremental AUC (iAUC) and outcome measures (markers of glycemic control and insulin sensitivity) were extracted. In case of missing data on exposures and outcomes, the authors were contacted to provide the required information. If the authors did not respond and relevant information was available in figures (i.e., bars for AUC, and responses per time point from graphs), data were extracted from figures using the Microsoft Excel add-in tool TM Image-to-data (tushar-mehta.com).
Quality assessment
Two reviewers (CR and MA) independently assessed the methodological quality of full-text papers using the Cochrane Risk of Bias Tool [12]. Differences in scores were resolved by consensus. Potential risk of bias was assessed by scoring seven different items (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, other sources of bias) with low, high or unclear risk of bias and is presented in Supplementary Figure 1.
Data synthesis and analysis
Outcome data were extracted if reported for at least five comparisons. The exposure and outcome measures glucose and insulin, with variance measure were transformed into SI units [mmol/l for glucose (= 0.0555 × mg/dl) and pmol/l for insulin (= 6 × microU/ml)].
In case postprandial responses were reported as data per time point (in table or as a figure), iAUCs were calculated by the trapezoidal method as net iAUC [13]. Relative changes in exposures PPG and PPI were calculated as:
The outcome was a mean difference between intervention and control. Baseline and post-intervention means with standard deviations (SD) or standard error of the mean (SEM) for the intervention and control groups were extracted, transforming SD into SEM (SEM = SD/√N, where N = subject population). When actual P values were reported, these were used to estimate the SEM [11]. In parallel studies, the absolute change in outcomes was calculated by subtracting the change from baseline in the control group from the change from baseline in the intervention group. In crossover studies, the post-intervention measure of the control group was subtracted from the post-intervention measure of the intervention group. The variance of the absolute changes in outcomes was calculated as (\(\sqrt {{\text{SE}}_{{{\text{intervention}}}}^{2} + {\text{SE}}_{{{\text{control}}}}^{2} }\)) for parallel studies and (\(\sqrt {{\text{SE}}_{{{\text{intervention}}\,{\text{end}}}}^{2} + {\text{SE}}_{{{\text{control}}\,{\text{end}}}}^{2} - 2r \times {\text{SE}}_{{{\text{intervention}}\,{\text{end}}}} \times {\text{SE}}_{{{\text{control}}\,{\text{end}}}} }\)) for crossover studies, assuming a within-subject correlation coefficient of 0.8.
Random effects meta-regression analyses were conducted (if number of comparisons k > 10) to estimate the association between changes in the acute PPG/PPI exposures and changes in longer-term risk factor outcomes. As additional analyses, overall effects on the acute postprandial exposures and on the outcome variables were estimated by meta-analyses and illustrated by forest plots. In these additional analyses, the postprandial exposures were expressed as mean postprandial levels, calculated as iAUC divided by time. The Q test (Chi2 statistic, P < 0.05) was used to evaluate between-study heterogeneity in meta-analysis and the residual heterogeneity in meta-regression analysis. The I2 statistic was used for quantification of the degree of heterogeneity and is interpretable as the percentage of the total association that may be due to heterogeneity between studies (I2 > 50% was considered a meaningful level of heterogeneity) in meta-analysis and as the residual heterogeneity in meta-regression analysis after correction for the changes in acute PPG/PPI exposures. The Pearson correlation coefficient between the change in PPG and the change in PPI was calculated. Bubble charts were created to visualize the relationship between the % relative change in PPG/PPI and the change in diabetes-related risk factors. Planned subgroup analyses stratified by normal versus abnormal glucose metabolism (non-diabetic hyperglycemia or diabetes) could not be conducted (because of k comparisons ≤ 10 per subgroup). Instead, for each comparison, normal versus abnormal glucose metabolism was marked by color in the bubble charts (abnormal glucose metabolism was defined on a study group level as being either impaired fasting glucose and/or impaired glucose tolerance and/or HbA1c > 5.7 (%) and/or use of glucose-lowering medication).
Meta-analysis was conducted in Review Manager (RevMan version 5.3. Copenhagen): The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Meta-regression analysis was performed in R version 3.4.2 using the Metafor package.
Results
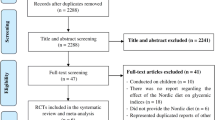
The search retrieved 13,004 papers and an additional 3 potentially relevant papers were found manually and added to the database for screening (Fig. 1). After removal of duplicates, 6964 papers were screened based on titles and abstracts; 146 full-text papers were finally assessed for eligibility. The main reasons for exclusion were: acute effects not reported (58 out of 128 excluded papers), not a PPG-lowering dietary intervention, and not a controlled trial. A total of 17 studies were eligible, of which 13 papers delivered all relevant data needed for quantitative analyses [14,15,16,17,18,19,20,21,22,23,24,25]. Three studies reported acute and chronic effects of the same dietary intervention in different papers [18, 26, 27] and [22, 28, 29]. One paper [21] reported data from two intervention and two control diets, thereby adding two independent comparisons. The total number of comparisons retrieved from the included set of papers for the quantitative analyses was 14. For PPG, there were 14 comparisons with outcome FPG, 12 with fasting insulin, and 7 with HbA1c. For PPI, there were 11 comparisons with outcome FPG, 10 for fasting insulin, and 4 for HbA1c.
Table 1 summarizes the characteristics of the studies included in the quantitative analyses. Two out of 14 comparisons aimed to reduce postprandial glucose via mulberry leaf extract supplementation [14, 19], while the other comparisons were dietary interventions of whole diet low GI (LGI) versus high GI (HGI) (6 comparisons), low GI breakfast (1 comparison), carbohydrate-reduced high-protein diet (1 comparison), type of rice (2 comparisons) and liquid carbohydrate-modified supplement (2 comparisons). At baseline, five comparisons (four studies) included individuals with normal glucose metabolism and nine comparisons included individuals with abnormal glucose metabolism. The study duration ranged from 4 weeks to 3 months. The intervention was applied to ≥ 3 main meals in nine comparisons, and to < 3 main meals in five comparisons. The duration of postprandial measurement ranged from 120 to 540 min, with a median and most frequent duration of 180 min.
The majority of the studies scored a high risk of bias on blinding of participants and personnel (Supplementary Fig. 1). All studies scored a low risk of bias on blinding of outcome assessment and selective reporting. Randomization and allocation concealment scored most frequently an unclear risk of bias.
The acute relative change in iAUC glucose ranged from − 121 to 3.5%, with a median of − 27.1%. The acute relative change in iAUC insulin ranged from − 36.8 to 33.2%, with a median of − 29.2%. The correlation between the change in iAUC glucose and the change in iAUC insulin was 0.69 (P = 0.019), see Supplementary Fig. 2.
Overall, the dietary interventions acutely reduced the absolute mean PPG levels (mean difference − 0.27 mmol/l; 95% CI − 0.41 to − 0.14; P < 0.0001; Supplementary Fig. 3A), but this effect was not significant for mean PPI level (mean difference − 7.47 pmol/l; 95% CI − 16.79 to 1.86; P = 0.12; Supplementary Fig. 3B).
No significant overall chronic effects were found for dietary intervention studies on fasting plasma glucose (mean difference 0.03 mmol/l; 95% CI − 0.27 to 0.33; P = 0.83) and fasting insulin (mean difference 3.10 pmol/l; 95% CI − 2.37 to 8.56; P = 0.27), but an overall reduction in HbA1c was observed (mean difference − 0.20%; 95% CI − 0.35 to − 0.05; P = 0.01) (Supplementary Fig. 4A–C).
The relationships between % relative acute changes in PPG/PPI and changes in FPG, fasting insulin, and HbA1c are presented in Fig. 2 and Supplementary Fig. 5. Three out of these six relationships had sufficient comparisons/data (k > 10) to conduct meta-regression analyses (Fig. 2). Changes in acute PPG responses were associated with changes in FPG (per 10% change in PPG: β = 0.085; 95% CI 0.003, 0.167; k = 14), but not with fasting insulin (β = 1.196; 95% CI − 0.321, 2.714; k = 12). Changes in acute PPI responses were not associated with changes in FPG (per 10% change in PPI: β = − 0.017; 95% CI − 0.056, 0.022; k = 11). By visual inspection, no differences in results were observed between studies with individuals with normal glucose metabolism versus studies with individuals with abnormal glucose metabolism (Fig. 2). Heterogeneity of all meta-analyses and meta-regression results was always below an I2 of 50% with the exception of the overall effects of the interventions on FPG (96%) and the association between acute PPG response and FPG (91.4%).
Bubble charts of the relationship between % relative change in PPG and absolute change in a FPG and b fasting insulin. c The relationship between % relative change in PPI and absolute change in FPG. The size of the bubbles indicates the weight of each study (inverse variance); aper 10% change in PPG; bper 10% change in PPI. Random effects meta-regression analyses were conducted (if number of comparisons k > 10) to estimate the association between changes in the acute PPG/PPI exposures and changes in longer-term risk factor outcomes. The I2 statistic was used for quantification of the degree of heterogeneity and is interpretable as the percentage of the total association that may be due to heterogeneity between studies (I2 > 50% was considered a meaningful level of heterogeneity) in meta-analysis and as the residual heterogeneity in meta-regression analysis after correction for the changes in acute PPG/PPI exposures. Bubble charts were created to visualize the relationship between the % relative change in PPG/PPI and the change in diabetes-related risk factors. For each comparison, normal versus abnormal glucose metabolism was marked by color in the bubble charts (abnormal glucose metabolism was defined on a study group level as being either impaired fasting glucose and/or impaired glucose tolerance and/or HbA1c > 5.7 (%) and/or use of glucose-lowering medication). Meta-regression analysis was performed in R version 3.4.2 using the Metafor package. FPG fasting plasma glucose, PPG postprandial glucose, PPI postprandial insulin
Discussion
This systematic review and meta-analysis of controlled dietary intervention studies aimed to investigate the size of the association between acute PPG and PPI responses and longer-term effects on diabetes-related risk factors. The evidence to examine this association was found to be limited to a set of 13 heterogeneous studies reporting 14 comparisons. An association was found between the size of the reduction in acute PPG exposures to study diets and FPG, but not between PPG and fasting insulin and HbA1c. No associations were found between acute PPI exposures and any of the outcomes.
A strength of this meta-analysis was the systematic approach to identify studies. Moreover, among included studies, the range in both PPG changes (− 121% to + 4%) and PPI changes (− 37% to + 33%) was substantial, which provided enough variation in exposures to potentially identify an association with outcomes. An important limitation was that our systematic review procedure yielded only a small number of studies that actually assessed PPG and PPI exposures to the diets under study. Most studies that aimed to reduce such exposures have designed the study diets based on published GI tables, or assumed effects on PPG, without quantification of actual PPG exposures, and were therefore not eligible for the present review. This perhaps identifies a limitation in the way nutritional research is currently undertaken. The small number of studies reduced study power and precluded analyses of effects on other outcomes (HbA1c). Another limitation of this meta-analysis is that the set of included studies were heterogeneous in study design and the number of studies did not allow for stratification by these sources of heterogeneity. Some major sources of potential heterogeneity were glucose metabolism status and the intensity of the intervention. Indeed, subjects with normal and abnormal glucose metabolism might respond differently to low GI interventions with a greater change in FPG reported previously in subjects with poor glycemic control [9]. The intensity of the intervention varied, as some involved all meals (whole diet approach) and others one meal only, which hampers quantification of PPG exposures during the day. Other potential sources of heterogeneity were study quality, duration of the chronic intervention and compliance to diets.
In our selected set of studies, a significant reduction in HbA1c, but no other longer-term risk factors (fasting glucose and insulin) following PPG-lowering dietary interventions of at least 4 weeks was found. These findings seem to be somewhat at odds with previous GI/GL epidemiologic and some intervention studies. Indeed, several prospective cohort studies have shown an association between GI/GL and the risk of T2D [30,31,32,33]. In a meta-analysis of prospective cohort studies, Barclay et al. concluded on an independent effect of GI/GL on the risk of developing T2D [34]. However, due to their observational nature, one cannot exclude the role of confounders (e.g., other dietary factors) in the observed association with T2D.
As reviewed by Blaak et al. results from short-term GI/GL intervention on insulin sensitivity and/or secretion still remain inconclusive [2]. While 11 studies demonstrated a beneficial effect on insulin sensitivity or insulin secretion, 10 papers did not report any difference. Livesey et al. performed a systematic review and meta-analysis of intervention trials on GI and markers of health [9]. They concluded on a favorable effect of consumption of reduced glycemic response diets on reduction of FPG and glycated proteins. However, the effect of low GI interventions seems to vary according to the subjects’ glucose control status. Indeed, the improvement in fasting blood glucose and glycated proteins was reported to be greater in subjects with poor fasting glucose control (> 5 mmol/l). Also, weak evidence suggested a reduction in fasting insulin concentration, only in people who were overweight or obese with fasting insulin concentrations above 100 pmol/l. We did not have sufficient data to tease out the differential effects between individuals with normal versus abnormal glucose metabolism, but the visual inspection did not indicate any differences between studies among individuals with normal versus abnormal glucose metabolism. The discrepancies with Livesey’s meta-analysis may be partially explained by the studies included [9]. Indeed, we only included studies in which the effect on the acute reductions of postprandial glycemia was quantified, while this effect was not assessed in most of the 45 publications included in Livesey et al.’s meta-analysis [9]. Despite the lack of overall effect on fasting glucose, the present study revealed a relationship of PPG with fasting plasma glucose. Given the heterogeneity of the studies and the lack of overall effect on fasting glucose, these results should be interpreted with care. On the other hand, our data do provide some support for a relationship between the intensity of the postprandial glucose response and that of the reduction in fasting glucose.
Although there is abundant evidence that elevated blood glucose, concomitantly with elevated insulin concentration, leads to a transitory deleterious metabolic and hormonal state and oxidative stress, involving the liver, the pancreas, skeletal muscles, lipid metabolism interactions as well as incretins and inflammatory parameters, the exact role of PPG and the relevant magnitude of effect in this process remains unknown [2]. However, it has been postulated that glycemic variability may be a much better indicator for related metabolic effects [35]. Indeed, multiple cohort studies have shown that a high glycemic variability is associated with an increased risk of cardiovascular disease in people with T2D independent of mean plasma glucose or HbA1c [36,37,38].
Daily exposures to glucose can currently be measured relatively non-invasively via continuous glucose monitoring (CGM) systems. In the present dataset, only one of the included studies utilized this system [28]. In an observational study that used CGM, a positive relationship between PPG and HbA1c was found, both in healthy individuals and those with diabetes yyyy [39]. Further application of CGM in (dietary) intervention studies that aim to reduce glycemic exposure would provide better understanding of achieved reductions in overall PPG exposure and variability. This will enable the estimation of relevant PPG reductions as well as setting benchmarks for PPG exposure in future interventions.
In conclusion, only a limited number of postprandial glucose-lowering dietary intervention studies measure the actual reductions in acute PPG/PPI to the intervention, which they then go on to administer chronically. In this small heterogeneous set of studies, an association was found between the magnitude of the acute postprandial responses and the change in fasting glucose but no other outcomes. To enable setting quantitative benchmarks for PPG/PPI reductions, future dietary intervention studies should consider measuring PPG/PPI exposure to study diets before embarking on a long-term dietary intervention. Similarly, investigators should be encouraged to move beyond the single acute meal study and to follow these up with a chronic intervention, to establish the true effects on metabolic risk.
Abbreviations
- BR:
-
Brown rice
- CGM:
-
Continuous glucose monitoring
- CHO:
-
Carbohydrate
- DNJ:
-
Deoxynojirimycin
- E:
-
Energy
- F:
-
Females
- FPG:
-
Fasting plasma glucose
- GBR:
-
Glutinous brown rice
- GI:
-
Glycemic index
- GL:
-
Glycemic load
- HGI:
-
High glycemic index
- HOMA-IR:
-
Homeostatic Model Assessment for Insulin Resistance
- iAUC:
-
Incremental area under the curve
- LGI:
-
Low glycemic index
- M:
-
Males
- MD:
-
Mean difference
- MLAE:
-
Mulberry leaf aqueous extract
- ONS:
-
Oral nutritional supplement
- PPG:
-
Postprandial glucose
- PPI:
-
Postprandial insulin
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- QUICKI:
-
Quantitative Insulin Sensitivity Check Index
- SD:
-
Standard deviation
- SEM:
-
Standard error of the mean
- T2D:
-
Type 2 diabetes
- WR:
-
White rice
References
International Diabetes Federation (2017) IDF Diabetes Atlas, 8th edn. International Diabetes Federation, Brussels, Belgium. https://www.diabetesatlas.org
Blaak EE, Antoine JM, Benton D, Bjorck I, Bozzetto L, Brouns F, Diamant M, Dye L, Hulshof T, Holst JJ, Lamport DJ, Laville M, Lawton CL, Meheust A, Nilson A, Normand S, Rivellese AA, Theis S, Torekov SS, Vinoy S (2012) Impact of postprandial glycaemia on health and prevention of disease. Obes Rev 13(10):923–984. https://doi.org/10.1111/j.1467-789X.2012.01011.x
Heianza Y, Hara S, Arase Y, Saito K, Fujiwara K, Tsuji H, Kodama S, Hsieh SD, Mori Y, Shimano H, Yamada N, Kosaka K, Sone H (2011) HbA1c 5.7–6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet 378(9786):147–155. https://doi.org/10.1016/S0140-6736(11)60472-8
Roumen C, Blaak EE, Corpeleijn E (2009) Lifestyle intervention for prevention of diabetes: determinants of success for future implementation. Nutr Rev 67(3):132–146. https://doi.org/10.1111/j.1753-4887.2009.00181.x
Samocha-Bonet D, Debs S, Greenfield JR (2018) Prevention and treatment of type 2 diabetes: a pathophysiological-based approach. Trends Endocrinol Metab 29(6):370–379. https://doi.org/10.1016/j.tem.2018.03.014
Verma S, Hussain ME (2017) Obesity and diabetes: an update. Diabetes Metab Syndrome 11(1):73–79. https://doi.org/10.1016/j.dsx.2016.06.017
Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, Diabetes Prevention Program Research G (2012) Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 379(9833):2243–2251. https://doi.org/10.1016/S0140-6736(12)60525-X
Clar C, Al-Khudairy L, Loveman E, Kelly SA, Hartley L, Flowers N, Germano R, Frost G, Rees K (2017) Low glycaemic index diets for the prevention of cardiovascular disease. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004467.pub3
Livesey G, Taylor R, Hulshof T, Howlett J (2008) Glycemic response and health—a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr 87(1):258S–268S. https://doi.org/10.1093/ajcn/87.1.258S
Zafar N, Krishnasamy SS, Shah J, Rai SN, Riggs DW, Bhatnagar A, O'Toole TE (2018) Circulating angiogenic stem cells in type 2 diabetes are associated with glycemic control and endothelial dysfunction. PLoS ONE 13(10):e0205851. https://doi.org/10.1371/journal.pone.0205851
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. https://handbook.cochrane.org
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, Wolever TM (2005) Glycaemic index methodology. Nutr Res Rev 18(1):145–171. https://doi.org/10.1079/NRR2005100
Asai A, Nakagawa K, Higuchi O, Kimura T, Kojima Y, Kariya J, Miyazawa T, Oikawa S (2011) Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J Diabetes Investig 2(4):318–323. https://doi.org/10.1111/j.2040-1124.2011.00101.x
Bouche C, Rizkalla SW, Luo J, Vidal H, Veronese A, Pacher N, Fouquet C, Lang V, Slama G (2002) Five-week, low-glycemic index diet decreases total fat mass and improves plasma lipid profile in moderately overweight nondiabetic men. Diabetes Care 25(5):822–828
Giacco R, Costabile G, Della Pepa G, Anniballi G, Griffo E, Mangione A, Cipriano P, Viscovo D, Clemente G, Landberg R, Pacini G, Rivellese AA, Riccardi G (2014) A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutr Metab Cardiovasc Dis 24(8):837–844. https://doi.org/10.1016/j.numecd.2014.01.007
Kabir M, Oppert JM, Vidal H, Bruzzo F, Fiquet C, Wursch P, Slama G, Rizkalla SW (2002) Four-week low-glycemic index breakfast with a modest amount of soluble fibers in type 2 diabetic men. Metabolism 51(7):819–826
Kallio P, Kolehmainen M, Laaksonen DE, Kekalainen J, Salopuro T, Sivenius K, Pulkkinen L, Mykkanen HM, Niskanen L, Uusitupa M, Poutanen KS (2007) Dietary carbohydrate modification induces alterations in gene expression in abdominal subcutaneous adipose tissue in persons with the metabolic syndrome: the FUNGENUT Study. Am J Clin Nutr 85(5):1417–1427. https://doi.org/10.1093/ajcn/85.5.1417
Kim JY, Ok HM, Kim J, Park SW, Kwon SW, Kwon O (2015) Mulberry leaf extract improves postprandial glucose response in prediabetic subjects: a randomized, double-blind placebo-controlled trial. J Med Food 18(3):306–313. https://doi.org/10.1089/jmf.2014.3160
Mayr P, Kuhn KS, Klein P, Stover JF, Pestana EA (2016) A diabetes-specific oral nutritional supplement improves glycaemic control in type 2 diabetes patients. Exp Clin Endocrinol Diabetes 124(7):401–409. https://doi.org/10.1055/s-0042-100909
McMillan-Price J, Petocz P, Atkinson F, O'Neill K, Samman S, Steinbeck K, Caterson I, Brand-Miller J (2006) Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med 166(14):1466–1475. https://doi.org/10.1001/archinte.166.14.1466
Nakayama T, Nagai Y, Uehara Y, Nakamura Y, Ishii S, Kato H, Tanaka Y (2017) Eating glutinous brown rice twice a day for 8 weeks improves glycemic control in Japanese patients with diabetes mellitus. Nutr Diabetes 7(5):e273. https://doi.org/10.1038/nutd.2017.26
Nazare JA, de Rougemont A, Normand S, Sauvinet V, Sothier M, Vinoy S, Desage M, Laville M (2010) Effect of postprandial modulation of glucose availability: short- and long-term analysis. Br J Nutr 103(10):1461–1470. https://doi.org/10.1017/S0007114509993357
Shimabukuro M, Higa M, Kinjo R, Yamakawa K, Tanaka H, Kozuka C, Yabiku K, Taira S, Sata M, Masuzaki H (2014) Effects of the brown rice diet on visceral obesity and endothelial function: the BRAVO study. Br J Nutr 111(2):310–320. https://doi.org/10.1017/S0007114513002432
Stenvers DJ, Schouten LJ, Jurgens J, Endert E, Kalsbeek A, Fliers E, Bisschop PH (2014) Breakfast replacement with a low-glycaemic response liquid formula in patients with type 2 diabetes: a randomised clinical trial. Br J Nutr 112(4):504–512. https://doi.org/10.1017/S0007114514001123
Kallio P, Kolehmainen M, Laaksonen DE, Pulkkinen L, Atalay M, Mykkanen H, Uusitupa M, Poutanen K, Niskanen L (2008) Inflammation markers are modulated by responses to diets differing in postprandial insulin responses in individuals with the metabolic syndrome. Am J Clin Nutr 87(5):1497–1503. https://doi.org/10.1093/ajcn/87.5.1497
Samkani A, Skytte MJ, Kandel D, Kjaer S, Astrup A, Deacon CF, Holst JJ, Madsbad S, Rehfeld JF, Haugaard SB, Krarup T (2018) A carbohydrate-reduced high-protein diet acutely decreases postprandial and diurnal glucose excursions in type 2 diabetes patients. Br J Nutr 119(8):910–917. https://doi.org/10.1017/S0007114518000521
Terashima Y, Nagai Y, Kato H, Ohta A, Tanaka Y (2017) Eating glutinous brown rice for one day improves glycemic control in Japanese patients with type 2 diabetes assessed by continuous glucose monitoring. Asia Pac J Clin Nutr 26(3):421–426. https://doi.org/10.6133/apjcn.042016.07
Skytte MJ, Samkani A, Petersen AD, Thomsen MN, Astrup A, Chabanova E, Frystyk J, Holst JJ, Thomsen HS, Madsbad S, Larsen TM, Haugaard SB, Krarup T (2019) A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: a randomised controlled trial. Diabetologia 62(11):2066–2078. https://doi.org/10.1007/s00125-019-4956-4
Livesey G, Taylor R, Livesey H, Liu S (2013) Is there a dose–response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am J Clin Nutr 97(3):584–596. https://doi.org/10.3945/ajcn.112.041467
Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC (1997) Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 20(4):545–550
Willett W, Manson J, Liu S (2002) Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr 76(1):274S–280S. https://doi.org/10.1093/ajcn/76/1.274S
Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB (2004) Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr 80(2):348–356. https://doi.org/10.1093/ajcn/80.2.348
Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC (2008) Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr 87(3):627–637. https://doi.org/10.1093/ajcn/87.3.627
Hall H, Perelman D, Breschi A, Limcaoco P, Kellogg R, McLaughlin T, Snyder M (2018) Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol 16(7):e2005143. https://doi.org/10.1371/journal.pbio.2005143
Bouchi R, Babazono T, Mugishima M, Yoshida N, Nyumura I, Toya K, Hayashi T, Hanai K, Tanaka N, Ishii A, Iwamoto Y (2012) Fluctuations in HbA1c are associated with a higher incidence of cardiovascular disease in Japanese patients with type 2 diabetes. J Diabetes Investig 3(2):148–155. https://doi.org/10.1111/j.2040-1124.2011.00155.x
Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, Mancia G, Poulter N, Harrap S, Woodward M, Chalmers J (2014) Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care 37(8):2359–2365. https://doi.org/10.2337/dc14-0199
Luk AO, Ma RC, Lau ES, Yang X, Lau WW, Yu LW, Chow FC, Chan JC, So WY (2013) Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong Diabetes Registry. Diabetes Metab Res Rev 29(5):384–390. https://doi.org/10.1002/dmrr.2404
Faerch K, Alssema M, Mela DJ, Borg R, Vistisen D (2018) Relative contributions of preprandial and postprandial glucose exposures, glycemic variability, and non-glycemic factors to HbA1c in individuals with and without diabetes. Nutr Diabetes 8(1):38. https://doi.org/10.1038/s41387-018-0047-8
Funding
This work was conducted by an expert group of the European branch of the International Life Sciences Institute, ILSI Europe. The Dietary Carbohydrates Task Force coordinated this publication. The industry members of this task force are listed on ILSI Europe’s website at https://ilsi.eu/task-forces/nutrition/dietary-carbohydrates/. Experts are not paid for the time spent on this work; however, the non-industry members within the expert group were offered support for travel and accommodation costs from the Dietary Carbohydrates Task Force to attend meetings to discuss the manuscript and a small compensatory honorarium with the option to decline. The expert group carried out the work, that is, collecting/analyzing data/information and writing the scientific paper separate from other activities of the task force. The research reported is the result of a scientific evaluation in line with ILSI Europe’s framework to provide a precompetitive setting for public–private partnership. ILSI Europe facilitated scientific meetings and coordinated the overall project management and administrative tasks related to the completion of this work. For further information about ILSI Europe, please email info@ilsieurope.be or call + 32-277-100-14. The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe nor those of its member companies or any regulatory authority.
Author information
Authors and Affiliations
Contributions
MA, EEB, LE, PD, SV, MDR: designed research (project conception, development of overall research plan, and study oversight): CR, MA, EEB, LE, PD, FS, SV, MDR: conducted research (hands-on conduct of the experiments and data collection) CR, MA, FS, SPR: analyzed data or performed statistical analysis and analyzed the extracted data; CR, MA, EEB, LE, SV, JWB, MDR: wrote the manuscript; CR, MA, JWB: had responsibility for final content. All authors edited and commented a version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
At the time of conducting the study, MA was an employee of Unilever, a manufacturer of consumer food products, LE was an employee of Nestec SA; and SV an employee of Mondēlez International R&D; CR, EEB, FS, JWB, JMD, PD, SPR and MDR have nothing to disclose. We thank Linda Schoonmade from VU University Amsterdam for designing and executing the literature search strategy. We also thank Femke Sijtsma and Jacqueline M. Dekker for their contribution.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruijgrok, C., Blaak, E.E., Egli, L. et al. Reducing postprandial glucose in dietary intervention studies and the magnitude of the effect on diabetes-related risk factors: a systematic review and meta-analysis. Eur J Nutr 60, 259–273 (2021). https://doi.org/10.1007/s00394-020-02240-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02240-1