Abstract
Purpose
Metabolic syndrome (MS) is a major public health issue worldwide and fructose consumption has been associated with MS development. Recently, we showed that the dietary polyphenol chrysin is an effective inhibitor of fructose uptake by human intestinal epithelial cells. Therefore, our aim was to investigate if chrysin interferes with the development of MS induced by fructose in an animal model.
Methods
Adult male Sprague–Dawley rats (220–310 g) were randomly divided into four groups: (A) tap water (control), (B) tap water and a daily dose of chrysin (100 mg/kg) by oral administration (chrysin) (C) 10% fructose in tap water (fructose), and (D) 10% fructose in tap water and a daily dose of chrysin (100 mg/kg) by oral administration (fructose + chrysin). All groups were fed ad libitum with standard laboratory chow diet and dietary manipulation lasted 18 weeks.
Results
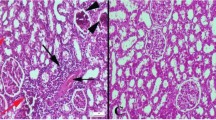
Fructose-feeding for 18 weeks induced an increase in serum triacylglycerols, insulin and angiotensin II levels and in hepatic fibrosis and these changes did not occur in fructose + chrysin rats. Moreover, the increase in both systolic and diastolic blood pressure which was found in fructose-fed animals from week 14th onwards was not observed in fructose + chrysin animals. In contrast, the increase in energy consumption, liver/body, heart/body and right kidney/body weight ratios, serum proteins, serum leptin and liver triacylglycerols observed in fructose-fed rats was not affected by chrysin.
Conclusions
Chrysin was able to protect against some of the MS features induced by fructose-feeding.







Similar content being viewed by others
Abbreviations
- MS:
-
Metabolic syndrome
- TAG:
-
Triacylglycerol
- FRUCT:
-
Fructose
- CHRYS:
-
Chrysin
- CV:
-
Cardiovascular diseases
- CONT:
-
Control
- OGTT:
-
Oral glucose tolerance test
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- MAP:
-
Mean arterial pressure
- HR:
-
Heart rate
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- ALP:
-
Alkaline phosphatase
- VLDL:
-
Very low-density lipoproteins
- LDL:
-
Low-density lipoprotein
- HDL:
-
high-density lipoproteins
- CRP:
-
C-reactive protein
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- mRNA:
-
Messenger ribonucleic acid
- NEFA:
-
Non-esterified fatty acids
- SREBP-1c:
-
Sterol regulatory binding protein 1c
- ChREBP:
-
Carbohydrate response element binding protein
- HMG-CoA:
-
3-Hidroxi-3-methyl-glutaril-CoA reductase
- NASH:
-
Non-alcoholic steatohepatitis
- AT1:
-
Angiotensin type 1
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate reductase
References
Alberti KG, Zimmet P, Shaw J (2006) Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 23(5):469–480. https://doi.org/10.1111/j.1464-5491.2006.01858.x
Wong SK, Chin KY, Suhaimi FH, Fairus A, Ima-Nirwana S (2016) Animal models of metabolic syndrome: a review. Nutr Metab (Lond) 13:65. https://doi.org/10.1186/s12986-016-0123-9
Boulange CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME (2016) Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med 8(1):42. https://doi.org/10.1186/s13073-016-0303-2
Yamanaka T, Fukuda T, Shirota S, Sawai Y, Murai T, Fujita N, Hosoi H (2013) The prevalence and characteristics of metabolic syndrome in patients with vertigo. PLoS One 8(12):e80176. https://doi.org/10.1371/journal.pone.0080176
Toop CR, Gentili S (2016) Fructose beverage consumption induces a metabolic syndrome phenotype in the rat: a systematic review and meta-analysis. Nutrients 8(9):577. https://doi.org/10.3390/nu8090577
Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K (2010) Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab 299(5):E685–E694. https://doi.org/10.1152/ajpendo.00283.2010
Tappy L, Le KA (2010) Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90(1):23–46. https://doi.org/10.1152/physrev.00019.2009
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47. https://doi.org/10.1017/jns.2016.41
Ramirez-Espinosa JJ, Saldana-Rios J, Garcia-Jimenez S, Villalobos-Molina R, Avila-Villarreal G, Rodriguez-Ocampo AN, Bernal-Fernandez G, Estrada-Soto S (2017) Chrysin induces antidiabetic, antidyslipidemic and anti-inflammatory effects in athymic nude diabetic mice. Molecules 23(1):E67. https://doi.org/10.3390/molecules23010067
Mani R, Natesan V (2018) Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 145:187–196. https://doi.org/10.1016/j.phytochem.2017.09.016
Andrade N, Araújo JR, Correia-Branco A, Carletti JV, Martel F (2017) Effect of dietary polyphenols on fructose uptake by human intestinal epithelial (Caco-2) cells. J Funct Foods 36:429–439. https://doi.org/10.1016/j.jff.2017.07.032
Jensen EC (2013) Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken) 296(3):378–381. https://doi.org/10.1002/ar.22641
Pereira CD, Severo M, Araujo JR, Guimaraes JT, Pestana D, Santos A, Ferreira R, Ascensao A, Magalhaes J, Azevedo I, Monteiro R, Martins MJ (2014) Relevance of a hypersaline sodium-rich naturally sparkling mineral water to the protection against metabolic syndrome induction in fructose-fed Sprague–Dawley rats: a biochemical, metabolic, and redox approach. Int J Endocrinol 2014:384583. https://doi.org/10.1155/2014/384583
Choi JH, Yun JW (2016) Chrysin induces brown fat-like phenotype and enhances lipid metabolism in 3T3-L1 adipocytes. Nutrition 32(9):1002–1010. https://doi.org/10.1016/j.nut.2016.02.007
El-Bassossy HM, Abo-Warda SM, Fahmy A (2013) Chrysin and luteolin attenuate diabetes-induced impairment in endothelial-dependent relaxation: effect on lipid profile, AGEs and NO generation. Phytother Res 27(11):1678–1684. https://doi.org/10.1002/ptr.4917
Li R, Zang A, Zhang L, Zhang H, Zhao L, Qi Z, Wang H (2014) Chrysin ameliorates diabetes-associated cognitive deficits in Wistar rats. Neurol Sci 35(10):1527–1532. https://doi.org/10.1007/s10072-014-1784-7
Rani N, Bharti S, Bhatia J, Nag TC, Ray R, Arya DS (2016) Chrysin, a PPAR-gamma agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem Biol Interact 250:59–67. https://doi.org/10.1016/j.cbi.2016.03.015
Samarghandian S, Azimi-Nezhad M, Samini F, Farkhondeh T (2016) Chrysin treatment improves diabetes and its complications in liver, brain, and pancreas in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol 94(4):388–393. https://doi.org/10.1139/cjpp-2014-0412
Singh J, Chaudhari BP, Kakkar P (2017) Baicalin and chrysin mixture imparts cyto-protection against methylglyoxal induced cytotoxicity and diabetic tubular injury by modulating RAGE, oxidative stress and inflammation. Environ Toxicol Pharmacol 50:67–75. https://doi.org/10.1016/j.etap.2017.01.013
Kang MK, Lee EJ, Kim YH, Kim DY, Oh H, Kim SI, Kang YH (2018) Chrysin ameliorates malfunction of retinoid visual cycle through blocking activation of AGE-RAGE-ER stress in glucose-stimulated retinal pigment epithelial cells and diabetic eyes. Nutrients 10(8):E1046. https://doi.org/10.3390/nu10081046
Kang MK, Park SH, Choi YJ, Shin D, Kang YH (2015) Chrysin inhibits diabetic renal tubulointerstitial fibrosis through blocking epithelial to mesenchymal transition. J Mol Med (Berl) 93(7):759–772. https://doi.org/10.1007/s00109-015-1301-3
Kang MK, Park SH, Kim YH, Lee EJ, Antika LD, Kim DY, Choi YJ, Kang YH (2016) Dietary compound chrysin inhibits retinal neovascularization with abnormal capillaries in db/db mice. Nutrients 8(12):E782. https://doi.org/10.3390/nu8120782
Kang MK, Park SH, Kim YH, Lee EJ, Antika LD, Kim DY, Choi YJ, Kang YH (2017) Chrysin ameliorates podocyte injury and slit diaphragm protein loss via inhibition of the PERK-eIF2alpha-ATF-CHOP pathway in diabetic mice. Acta Pharmacol Sin 38(8):1129–1140. https://doi.org/10.1038/aps.2017.30
Lee EJ, Kang MK, Kim DY, Kim YH, Oh H, Kang YH (2018) Chrysin inhibits advanced glycation end products-induced kidney fibrosis in renal mesangial cells and diabetic kidneys. Nutrients 10(7):E882. https://doi.org/10.3390/nu10070882
Ahad A, Ganai AA, Mujeeb M, Siddiqui WA (2014) Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol Appl Pharmacol 279(1):1–7. https://doi.org/10.1016/j.taap.2014.05.007
Sirovina D, Orsolic N, Koncic MZ, Kovacevic G, Benkovic V, Gregorovic G (2013) Quercetin vs chrysin: effect on liver histopathology in diabetic mice. Hum Exp Toxicol 32(10):1058–1066. https://doi.org/10.1177/0960327112472993
El-Bassossy HM, Abo-Warda SM, Fahmy A (2014) Chrysin and luteolin alleviate vascular complications associated with insulin resistance mainly through PPAR-gamma activation. Am J Chin Med 42(5):1153–1167. https://doi.org/10.1142/S0192415X14500724
Litterio MC, Vazquez Prieto MA, Adamo AM, Elesgaray R, Oteiza PI, Galleano M, Fraga CG (2015) (−)-Epicatechin reduces blood pressure increase in high-fructose-fed rats: effects on the determinants of nitric oxide bioavailability. J Nutr Biochem 26(7):745–751. https://doi.org/10.1016/j.jnutbio.2015.02.004
Novak A, Godoy YC, Martinez SA, Ghanem CI, Celuch SM (2015) Fructose-induced metabolic syndrome decreases protein expression and activity of intestinal P-glycoprotein. Nutrition 31(6):871–876. https://doi.org/10.1016/j.nut.2015.01.003
Ge CX, Yu R, Xu MX, Li PQ, Fan CY, Li JM, Kong LD (2016) Betaine prevented fructose-induced NAFLD by regulating LXRalpha/PPARalpha pathway and alleviating ER stress in rats. Eur J Pharmacol 770:154–164. https://doi.org/10.1016/j.ejphar.2015.11.043
Kamide K, Rakugi H, Higaki J, Okamura A, Nagai M, Moriguchi K, Ohishi M, Satoh N, Tuck ML, Ogihara T (2002) The renin–angiotensin and adrenergic nervous system in cardiac hypertrophy in fructose-fed rats. Am J Hypert 15(1 Pt 1):66–71. https://doi.org/10.1016/s0895-7061(01)02232-4
Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K, Hayashi S, Yamaji R, Inui H, Fukusato T, Yamanouchi T (2009) Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr 139(11):2067–2071. https://doi.org/10.3945/jn.109.105858
Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM, Nonalcoholic Steatohepatitis Clinical Research N (2010) Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51(6):1961–1971. https://doi.org/10.1002/hep.23535
Vila L, Rebollo A, Adalsteisson GS, Alegret M, Merlos M, Roglans N, Laguna JC (2011) Reduction of liver fructokinase expression and improved hepatic inflammation and metabolism in liquid fructose-fed rats after atorvastatin treatment. Toxicol Appl Pharmacol 251(1):32–40. https://doi.org/10.1016/j.taap.2010.11.011
Catena C, Giacchetti G, Novello M, Colussi G, Cavarape A, Sechi LA (2003) Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension*. Am J Hypertens 16(11):973–978. https://doi.org/10.1016/S0895-7061(03)01002-1
Le KA, Tappy L (2006) Metabolic effects of fructose. Curr Opin Clin Nutr Metab Care 9(4):469–475. https://doi.org/10.1097/01.mco.0000232910.61612.4d
Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ (2008) Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol 295(5):R1370–R1375. https://doi.org/10.1152/ajpregu.00195.2008
Knight ZA, Hannan KS, Greenberg ML, Friedman JM (2010) Hyperleptinemia is required for the development of leptin resistance. PLoS One 5(6):e11376. https://doi.org/10.1371/journal.pone.0011376
Roglans N, Vila L, Farre M, Alegret M, Sanchez RM, Vazquez-Carrera M, Laguna JC (2007) Impairment of hepatic Stat-3 activation and reduction of PPARalpha activity in fructose-fed rats. Hepatology 45(3):778–788. https://doi.org/10.1002/hep.21499
Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, Schwartz GJ, Kunos G, Rossetti L, Buettner C (2008) Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med 14(6):667–675. https://doi.org/10.1038/nm1775
Lustig RH, Sen S, Soberman JE, Velasquez-Mieyer PA (2004) Obesity, leptin resistance, and the effects of insulin reduction. Int J Obes 28:1344. https://doi.org/10.1038/sj.ijo.0802753
Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G, Sela BA (2005) Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension 45(5):1012–1018. https://doi.org/10.1161/01.HYP.0000164570.20420.67
Bursac BN, Djordjevic AD, Vasiljevic AD, Milutinovic DD, Velickovic NA, Nestorovic NM, Matic GM (2013) Fructose consumption enhances glucocorticoid action in rat visceral adipose tissue. J Nutr Biochem 24(6):1166–1172. https://doi.org/10.1016/j.jnutbio.2012.09.002
Koo HY, Wallig MA, Chung BH, Nara TY, Cho BH, Nakamura MT (2008) Dietary fructose induces a wide range of genes with distinct shift in carbohydrate and lipid metabolism in fed and fasted rat liver. Biochim Biophys Acta 1782(5):341–348. https://doi.org/10.1016/j.bbadis.2008.02.007
Samuel VT (2011) Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab 22(2):60–65. https://doi.org/10.1016/j.tem.2010.10.003
Prince PD, Lanzi CR, Toblli JE, Elesgaray R, Oteiza PI, Fraga CG, Galleano M (2016) Dietary (−)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radic Biol Med 90:35–46. https://doi.org/10.1016/j.freeradbiomed.2015.11.009
Khanal RC, Howard LR, Wilkes SE, Rogers TJ, Prior RL (2012) Effect of dietary blueberry pomace on selected metabolic factors associated with high fructose feeding in growing Sprague–Dawley rats. J Med Food 15(9):802–810. https://doi.org/10.1089/jmf.2011.0212
Zarzecki MS, Araujo SM, Bortolotto VC, de Paula MT, Jesse CR, Prigol M (2014) Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol Rep 1:200–208. https://doi.org/10.1016/j.toxrep.2014.02.003
Ichimura M, Masuzumi M, Kawase M, Sakaki M, Tamaru S, Nagata Y, Tanaka K, Suruga K, Tsuneyama K, Matsuda S, Omagari K (2017) A diet-induced Sprague–Dawley rat model of nonalcoholic steatohepatitis-related cirrhosis. J Nutr Biochem 40:62–69. https://doi.org/10.1016/j.jnutbio.2016.10.007
Jeyapal S, Putcha UK, Mullapudi VS, Ghosh S, Sakamuri A, Kona SR, Vadakattu SS, Madakasira C, Ibrahim A (2018) Chronic consumption of fructose in combination with trans fatty acids but not with saturated fatty acids induces nonalcoholic steatohepatitis with fibrosis in rats. Eur J Nutr 57(6):2171–2187. https://doi.org/10.1007/s00394-017-1492-1
Softic S, Cohen DE, Kahn CR (2016) Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci 61(5):1282–1293. https://doi.org/10.1007/s10620-016-4054-0
Balta C, Ciceu A, Herman H, Rosu M, Boldura OM, Hermenean A (2018) Dose-dependent antifibrotic effect of chrysin on regression of liver fibrosis: the role in extracellular matrix remodeling. Dose Response 16(3):1559325818789835. https://doi.org/10.1177/1559325818789835
Hermenean A, Mariasiu T, Navarro-Gonzalez I, Vegara-Meseguer J, Miutescu E, Chakraborty S, Perez-Sanchez H (2017) Hepatoprotective activity of chrysin is mediated through TNF-alpha in chemically-induced acute liver damage: an in vivo study and molecular modeling. Exp Ther Med 13(5):1671–1680. https://doi.org/10.3892/etm.2017.4181
Klein AV, Kiat H (2015) The mechanisms underlying fructose-induced hypertension: a review. J Hypertens 33(5):912–920. https://doi.org/10.1097/HJH.0000000000000551
Ramanathan V, Thekkumalai M (2014) Role of chrysin on hepatic and renal activities of Nω-nitro-l-arginine-methylester induced hypertensive rats. Int J Nutr Pharmacol Neurol Dis 4(1):58–63. https://doi.org/10.4103/2231-0738.124615
Veerappan R, Malarvili T (2018) Chrysin pretreatment improves angiotensin system, cGMP concentration in L-NAME induced hypertensive rats. Indian J Clin Biochem. https://doi.org/10.1007/s12291-018-0761-y
Gordish KL, Kassem KM, Ortiz PA, Beierwaltes WH (2017) Moderate (20%) fructose-enriched diet stimulates salt-sensitive hypertension with increased salt retention and decreased renal nitric oxide. Physiol Rep 5(7):pii: e13162. https://doi.org/10.14814/phy2.13162
Shinozaki K, Ayajiki K, Nishio Y, Sugaya T, Kashiwagi A, Okamura T (2004) Evidence for a causal role of the renin–angiotensin system in vascular dysfunction associated with insulin resistance. Hypertension 43(2):255–262. https://doi.org/10.1161/01.HYP.0000111136.86976.26
Hitomi H, Kiyomoto H, Nishiyama A (2007) Angiotensin II and oxidative stress. Curr Opin Cardiol 22(4):311–315. https://doi.org/10.1097/HCO.0b013e3281532b53
Cai H (2005) NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res 96(8):818–822. https://doi.org/10.1161/01.RES.0000163631.07205.fb
Mayer MA, Höcht C, Giani JF, Muñoz MC, Carranza A, Taira CA, Dominici FP, Puyó AM, Fernández BE (2013) Central insulin–angiotensin II interaction in blood pressure regulation in fructose overloaded rats. Regul Pept 185:37–43. https://doi.org/10.1016/j.regpep.2013.06.001
Bundalo MM, Zivkovic MD, Romic SD, Tepavcevic SN, Koricanac GB, Djuric TM, Stankovic AD (2016) Fructose-rich diet induces gender-specific changes in expression of the renin–angiotensin system in rat heart and upregulates the ACE/AT1R axis in the male rat aorta. J Renin Angiotensin Aldosterone Syst 17(2):1470320316642915. https://doi.org/10.1177/1470320316642915
Xu X, Tu L, Wang L, Fang X, Wang DW (2011) CYP2J3 gene delivery reduces insulin resistance via upregulation of eNOS in fructose-treated rats. Cardiovasc Diabetol 10:114. https://doi.org/10.1186/1475-2840-10-114
Villar IC, Jimenez R, Galisteo M, Garcia-Saura MF, Zarzuelo A, Duarte J (2002) Effects of chronic chrysin treatment in spontaneously hypertensive rats. Planta Med 68(9):847–850. https://doi.org/10.1055/s-2002-34400
Denver RJ, Bonett RM, Boorse GC (2011) Evolution of leptin structure and function. Neuroendocrinology 94(1):21–38. https://doi.org/10.1159/000328435
Harwood HJ Jr (2012) The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 63(1):57–75. https://doi.org/10.1016/j.neuropharm.2011.12.010
Zamami Y, Takatori S, Hobara N, Yabumae N, Tangsucharit P, Jin X, Hashikawa N, Kitamura Y, Sasaki K, Kawasaki H (2011) Hyperinsulinemia induces hypertension associated with neurogenic vascular dysfunction resulting from abnormal perivascular innervations in rat mesenteric resistance arteries. Hypertens Res 34(11):1190–1196. https://doi.org/10.1038/hr.2011.97
Samarghandian S, Farkhondeh T, Azimi-Nezhad M (2017) Protective effects of chrysin against drugs and toxic agents. Dose Response 15(2):1559325817711782. https://doi.org/10.1177/1559325817711782
Acknowledgements
We thank animal facility crew from Faculty of Medicine of the University of Porto for all technical support.
Funding
This work was financed by CAPES—Brazilian Federal Agency for Support and Evaluation of Graduate Education within the Ministry of Education of Brazil, for financing this project—PN: 10103/13-9.
Author information
Authors and Affiliations
Contributions
Conception and design: NA and FM. Acquisition of data: NA. Technical support: JTG, IR, LG. Analysis and interpretation of data: NA, FM, EK, SA, CS. Drafting the article and revising it for intellectual content: NA, FM. Study Supervision: FM. Final approval of the completed article: NA, FM, EK, SA, CS, JTG, IR, LG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Andrade, N., Andrade, S., Silva, C. et al. Chronic consumption of the dietary polyphenol chrysin attenuates metabolic disease in fructose-fed rats. Eur J Nutr 59, 151–165 (2020). https://doi.org/10.1007/s00394-019-01895-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-01895-9