Abstract
Purpose
Consumption of Western diet high in fat and fructose has been attributed to the recent epidemic of nonalcoholic fatty liver disease (NAFLD). However, the impact of specific fatty acids on the progression of NAFLD to nonalcoholic steatohepatitis (NASH) is poorly understood. In the present study, we investigated the chronic effects of consumption of fructose in combination with saturated fatty acids (SFA) or trans fatty acids (TFA) on the development of NAFLD.
Methods
Male Sprague–Dawley rats were randomly assigned to six isocaloric starch/high fructose (44% of calories), high fat (39% calories) diet containing either starch–peanut oil, fructose–peanut oil, fructose–palmolein, fructose–clarified butter, fructose–coconut oil or fructose–partially hydrogenated vegetable oil and fed for 24 weeks. Palmolein, clarified butter and coconut oil were used as the source of SFA whereas partially hydrogenated vegetable oil was used as the source of TFA. Peanut oil was used as the reference oil.
Results
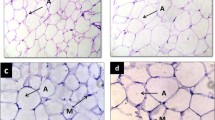
Long-term feeding of fructose in combination with SFA or TFA induced hepatic steatosis of similar extent associated with upregulation of stearoyl CoA desaturase-1. In contrast, fructose in combination with TFA induced NASH with fibrosis as evidenced by upregulation of hepatic proinflammatory cytokine and fibrogenic gene expression, increased hepatic oxidative stress and adipocytokine imbalance. Histopathological analysis revealed the presence of NASH with fibrosis. Further, peanut oil prevented the development of NAFLD in fructose-fed rats.
Conclusion
Fructose in combination with TFA caused NASH with fibrosis by inducing oxidative stress and inflammation, whereas, fructose in combination with SFA caused simple steatosis, suggesting that the type of fatty acid is more important for the progression of NAFLD.






Similar content being viewed by others
Abbreviations
- ACC α:
-
Acetyl CoA carboxylase alpha
- ALT:
-
Alanine aminotransferases
- AST:
-
Aspartate aminotransferases
- AUC:
-
Area under the curve
- CB:
-
Clarified butter
- ChREBP:
-
Carbohydrate responsive element binding protein
- CO:
-
Coconut oil
- CPT 1:
-
Carnitine palmotyl tranferase 1
- ELISA:
-
Enzyme linked immunosorbent assay
- FAME:
-
Fatty acid methyl ester
- FAS:
-
Fatty acid synthase
- FR-PNO:
-
Fructose–peanut oil
- FR-PO:
-
Fructose–palmolein
- FR-CB:
-
Fructose–clarified butter
- FR-CO:
-
Fructose–coconut oil
- FR-PHVO:
-
Fructose–partially hydrogenated vegetable oil
- GPx:
-
Glutathione peroxidase
- GSH:
-
Reduced glutathione
- H&E:
-
Hematoxylin and eosin
- HDL:
-
High-density lipoprotein
- HOMA-IR:
-
Homeostasis model assessment-insulin resistance
- HSC:
-
Hepatic stellate cells
- IL-1β:
-
Interleukin-1 beta
- IL-6:
-
Interleukin-6
- IPGTT:
-
Intraperitoneal glucose tolerance test
- NAFLD:
-
Nonalcoholic fatty liver disease
- NAS:
-
NAFLD Activity score
- NASH:
-
Nonalcoholic steatohepatitis
- PAI-1:
-
Plasminogen activator inhibitor-1
- PCR:
-
Polymerase chain reaction
- PHVO:
-
Partially hydrogenated vegetable oil
- PNO:
-
Peanut oil
- PO:
-
Palmolein
- PPAR α:
-
Peroxisome proliferator-activated receptor alpha
- PPAR γ:
-
Peroxisome proliferator-activated receptor gamma
- SCD-1:
-
Stearoyl CoA desaturase-1
- SFA:
-
Saturated fatty acid
- SOD:
-
Superoxide dismutase
- SREBP 1c:
-
Sterol regulatory element binding protein 1c
- TBARS:
-
Thiobarbituric acid reactive substances
- TFA:
-
Trans fatty acid
- TNF α:
-
Tumor necrosis factor alpha
References
Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE (2013) From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol 10:627–636. doi:10.1038/nrgastro.2013.149
Bedossa P (2017) Pathology of non-alcoholic fatty liver disease. Liver Int 37:85–89. doi:10.1111/liv.13301
Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK et al (2011) Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 141:1249–1253. doi:10.1053/j.gastro.2011.06.061
Buzzetti E, Pinzani M, Tsochatzis EA (2016) The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65:1038–1048. doi:10.1016/j.metabol.2015.12.012
Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM (2016) Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 65:1017–1025. doi:10.1016/j.metabol.2016.01.012
Amarapurkar D, Kamani P, Patel NP, Kumar P, Agal S et al (2007) Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol 6:161–163
Mohan V, Farooq S, Deepa M, Ravikumar R, Pitchumoni CS (2009) Prevalence of non-alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract 84:84–91. doi:10.1016/j.diabres.2008.11.039
Asrih M, Jornayvaz FR (2015) Metabolic syndrome and nonalcoholic fatty liver disease: is insulin resistance the link? Mol Cell Endocrinol 1:55–65. doi:10.1016/j.mce.2015.02.018
Ibrahim A, Natrajan S, Ghafoorunissa R (2005) Dietary trans-fatty acids alter adipocyte plasma membrane fatty acid composition and insulin sensitivity in rats. Metabolism 54:240–246
Natarajan S, Ibrahim A, Ghafoorunissa (2005) Dietary trans fatty acids alter diaphragm phospholipid fatty acid composition, triacylglycerol content and glucose transport in rats. Br J Nutr 93:829–833. doi:10.1079/BJN20051442
Oddy WH, Herbison CE, Jacoby P, Ambrosini GL, O’Sullivan TA et al (2013) The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am J Gastroenterol 108:778–785. doi:10.1038/ajg.2013.95
Asrih M, Jornayvaz FR (2014) Diets and nonalcoholic fatty liver disease: the good and the bad. Clin Nutr 33:186–190. doi:10.1016/j.clnu.2013.11.003
Perito ER, Rodriguez LA, Lustig RH (2013) Dietary treatment of nonalcoholic steatohepatitis. Curr Opin Gastroenterol 29:170–176. doi:10.1097/MOG.0b013e32835ca11d
Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH (2010) The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 7:251–264. doi:10.1038/nrgastro.2010.41
Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI et al (2007) Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86:899–906
Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K et al (2009) Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr 139:2067–2071. doi:10.3945/jn.109.105858
Sakamuri A, Pitla S, Putcha UK, Jayapal S, Pothana S et al (2016) Transient decrease in circulatory testosterone and homocysteine precedes the development of metabolic syndrome features in fructose-fed Sprague Dawley rats. J Nutr Metab 2016:7510840. doi:10.1155/2016/7510840
Oliveira LS, Santos DA, Barbosa-da-Silva S, Mandarim-de-Lacerda CA, Aguila MB (2014) The inflammatory profile and liver damage of a sucrose-rich diet in mice. J Nutr Biochem 25:193–200. doi:10.1016/j.jnutbio.2013.10.006
Mock K, Lateef S, Benedito VA, Tou JC (2017) High-fructose corn syrup-55 consumption alters hepatic lipid metabolism and promotes triglyceride accumulation. J Nutr Biochem 39:32–39. doi:10.1016/j.jnutbio.2016.09.010
Romestaing C, Piquet MA, Bedu E, Rouleau V, Dautresme M et al (2007) Long term highly saturated fat diet does not induce NASH in Wistar rats. Nutr Metab (Lond) 4:4. doi:10.1186/1743-7075-4-4
Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA et al (2006) Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol 36:485–501. doi:10.1677/jme.1.01909
Panchal SK, Poudyal H, Iyer A, Nazer R, Alam MA et al (2011) High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol 57:611–624. doi:10.1097/FJC.0b013e31821b1379
Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ et al (2013) High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 58:1632–1643. doi:10.1002/hep.26594
Tetri LH, Basaranoglu M, Brunt EM, Yerian LM, Neuschwander-Tetri BA (2008) Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol 295:G987–G995. doi:10.1152/ajpgi.90272.2008
Mells JE, Fu PP, Kumar P, Smith T, Karpen SJ et al (2015) Saturated fat and cholesterol are critical to inducing murine metabolic syndrome with robust nonalcoholic steatohepatitis. J Nutr Biochem 26:285–292. doi:10.1016/j.jnutbio.2014.11.002
Ichimura M, Kawase M, Masuzumi M, Sakaki M, Nagata Y et al (2015) High-fat and high-cholesterol diet rapidly induces non-alcoholic steatohepatitis with advanced fibrosis in Sprague–Dawley rats. Hepatol Res 45:458–469. doi:10.1111/hepr.12358
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Ghafoorunissa, Reddy V, Sesikaran B (1995) Palmolein and groundnut oil have comparable effects on blood lipids and platelet aggregation in healthy Indian subjects. Lipids 30:1163–1169. doi:10.1007/BF02536619
Huang LL, Wan JB, Wang B, He CW, Ma H et al (2013) Suppression of acute ethanol-induced hepatic steatosis by docosahexaenoic acid is associated with downregulation of stearoyl-CoA desaturase 1 and inflammatory cytokines. Prostaglandins Leukot Essent Fatty Acids 88:347–353. doi:10.1016/j.plefa.2013.02.002
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113:548–555. doi:10.1016/S0076-6879(85)13073-9
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. doi:10.1139/o59-099
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Straub BK, Schirmacher P (2010) Pathology and biopsy assessment of non-alcoholic fatty liver disease. Dig Dis 28:197–202. doi:10.1159/000282086
Schindhelm RK, Diamant M, Dekker JM, Tushuizen ME, Teerlink T et al (2006) Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev 22:437–443. doi:10.1002/dmrr.666
Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A et al (2008) Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology 48:792–798. doi:10.1002/hep.22429
Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA et al (2003) Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 37:1286–1292. doi:10.1053/jhep.2003.50229
Kawano Y, Cohen DE (2013) Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol 48:434–441. doi:10.1007/s00535-013-0758-5
Softic S, Cohen DE, Kahn CR (2016) Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci 61:1282–1293. doi:10.1007/s10620-016-4054-0
Dentin R, Girard J, Postic C (2005) Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie 87:81–86. doi:10.1016/j.biochi.2004.11.008
Narce M, Bellenger J, Rialland M, Bellenger S (2012) Recent advances on stearoyl-CoA desaturase regulation in fatty liver diseases. Curr Drug Metab 13:1454–1463. doi:10.2174/138920012803762693
Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM (2000) The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem 275:30132–30138. doi:10.1074/jbc.M005488200
Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H et al (2004) Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem 279:25164–25171. doi:10.1074/jbc.M402781200
Liu L, Wang S, Yao L, Li JX, Ma P et al (2016) Long-term fructose consumption prolongs hepatic stearoyl-CoA desaturase 1 activity independent of upstream regulation in rats. Biochem Biophys Res Commun 479:643–648. doi:10.1016/j.bbrc.2016.09.160
Sellmann C, Priebs J, Landmann M, Degen C, Engstler AJ et al (2015) Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J Nutr Biochem 26:1183–1192. doi:10.1016/j.jnutbio.2015.05.011
Lee JS, Jun DW, Kim EK, Jeon HJ, Nam HH (2015) Histologic and metabolic derangement in high-fat, high-fructose, and combination diet animal models. Sci World J 2015:306326. doi:10.1155/2015/306326
Pierce AA, Duwaerts CC, Soon RK, Siao K, Grenert JP et al (2016) Isocaloric manipulation of macronutrients within a high-carbohydrate/moderate-fat diet induces unique effects on hepatic lipogenesis, steatosis and liver injury. J Nutr Biochem 29:12–20. doi:10.1016/j.jnutbio.2015.10.020
de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J, Anand SS (2015) Intake of saturated and trans unsaturated fatty acids and risk of all-cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 351:h3978. doi:10.1136/bmj.h3978
Obara N, Fukushima K, Ueno Y, Wakui Y, Kimura O et al (2010) Possible involvement and the mechanisms of excess trans-fatty acid consumption in severe NAFLD in mice. J Hepatol 53:326–334. doi:10.1016/j.jhep.2010.02.029
Machado RM, Stefano JT, Oliveira CP, Mello ES, Ferreira FD et al (2010) Intake of trans fatty acids causes nonalcoholic steatohepatitis and reduces adipose tissue fat content. J Nutr 140:1127–1132. doi:10.3945/jn.109.117937
Hu X, Tanaka N, Guo R, Lu Y, Nakajima T et al (2017) PPARα protects against trans-fatty-acid-containing diet-induced steatohepatitis. J Nutr Biochem 39:77–85. doi:10.1016/j.jnutbio.2016.09.015
Kondoh Y, Kawada T, Urade R (2007) Activation of caspase 3 in HepG2 cells by elaidic acid (t18:1). Biochim Biophys Acta 1771:500–505. doi:10.1016/j.bbalip.2007.01.012
Rolo AP, Teodoro JS, Palmeira CM (2012) Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med 52:59–69. doi:10.1016/j.freeradbiomed.2011.10.003
Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN et al (2008) Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 103:1372–1379. doi:10.1111/j.1572-0241.2007.01774.x
Coulon S, Francque S, Colle I, Verrijken A, Blomme B et al (2012) Evaluation of inflammatory and angiogenic factors in patients with non-alcoholic fatty liver disease. Cytokine 59:442–449. doi:10.1016/j.cyto.2012.05.001
Polyzos SA, Kountouras J, Mantzoros CS (2016) Adipokines in nonalcoholic fatty liver disease. Metabolism 65:1062–1079. doi:10.1016/j.metabol.2015.11.006
Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A et al (2005) Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab 90:3498–3504. doi:10.1210/jc.2004-2240
Polyzos SA, Aronis KN, Kountouras J, Raptis DD, Vasiloglou MF et al (2016) Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia 59:30–43. doi:10.1007/s00125-015-3769-3
Nieto N, Friedman SL, Greenwel P, Cederbaum AI (1999) CYP2E1-mediated oxidative stress induces collagen type I expression in rat hepatic stellate cells. Hepatology 30:987–996. doi:10.1002/hep.510300433
Tang Y, Zheng S, Chen A (2009) Curcumin eliminates leptin’s effects on hepatic stellate cell activation via interrupting leptin signaling. Endocrinology 150:3011–3020. doi:10.1210/en.2008-1601
Andrade JM, Paraíso AF, de Oliveira MV, Martins AM, Neto JF et al (2014) Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition 30:915–919. doi:10.1016/j.nut.2013.11.016
Chen S, Zhao X, Ran L, Wan J, Wang X et al (2015) Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis 47:226–232. doi:10.1016/j.dld.2014.11.015
Ma F, Li P, Zhang Q, Yu L, Zhang L (2015) Rapid determination of trans-resveratrol in vegetable oils using magnetic hydrophilic multi-walled carbon nanotubes as adsorbents followed by liquid chromatography-tandem mass spectrometry. Food Chem 178:259–266. doi:10.1016/j.foodchem.2015.01.021
Acknowledgements
This study was funded by Grants in aid (5/4/3-7/TF/2011/NCD-II) from Indian Council of Medical Research, Government of India to AI. JS was supported by a fellowship from Indian Council of Medical Research, Government of India.
Author information
Authors and Affiliations
Contributions
AI and JS designed the study, analyzed the data and wrote the manuscript. JS, AS, KSR and VSS conducted the animal experiment and prepared the experimental diets. PUK and MVS carried out histopathological analysis of liver and NAS scoring. SG and JS involved in mRNA expression studies by RT-qPCR. JS, KSR and CM carried out biochemical estimations. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jeyapal, S., Putcha, U.K., Mullapudi, V.S. et al. Chronic consumption of fructose in combination with trans fatty acids but not with saturated fatty acids induces nonalcoholic steatohepatitis with fibrosis in rats. Eur J Nutr 57, 2171–2187 (2018). https://doi.org/10.1007/s00394-017-1492-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1492-1