Abstract
Purpose
The anti-inflammatory activity of sardine protein hydrolysates (SPH) obtained by hydrolysis with proteases from brewing yeast surplus was ascertained.
Methods
For this purpose, a digested and desalted SPH fraction with molecular weight lower than 10 kDa was investigated using an endothelial cell line (EA.hy926) as such and in a co-culture model with an intestinal cell line (Caco-2). Effects of SPH <10 kDa on nitric oxide (NO) production, reactive oxygen species (ROS) inhibition and secretion of monocyte chemoattractant protein 1 (MCP-1), vascular endothelial growth factor (VEGF), chemokine IL-8 (IL-8) and intercellular adhesion molecule-1 (ICAM-1) were evaluated in TNF-α-treated and untreated cells.
Results
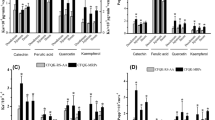
Upon TNF-α treatment, levels of NO, MCP-1, VEGF, IL-8, ICAM-1 and endothelial ROS were significantly increased in both mono- and co-culture models. Treatment with SPH <10 kDa (2.0 mg peptides/mL) significantly decreased all the inflammation markers when compared to TNF-α-treated control. This protective effect was more pronounced in the co-culture model, suggesting that SPH <10 kDa Caco-2 cells metabolites produced in the course of intestinal absorption may provide a more relevant protective effect against endothelial dysfunction. Additionally, indirect cross-talk between two cell types was established, suggesting that SPH <10 kDa may also bind to receptors on the Caco-2 cells, thereby triggering a pathway to secrete the pro-inflammatory compounds.
Conclusion
Overall, these in vitro screening results, in which intestinal digestion, absorption and endothelial bioactivity are simulated, show the potential of SPH to be used as a functional food with anti-inflammatory properties.






Similar content being viewed by others
Abbreviations
- SPH:
-
Sardine protein hydrolysate
- GI:
-
Gastrointestinal
- TNF-α:
-
Tumor necrosis factor-α
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
- ICAM-1:
-
Intercellular adhesion molecule-1
- IL-8:
-
Interleukin-8
- MCP-1:
-
Monocyte chemoattractant protein 1
- VEGF:
-
Vascular endothelial growth factor
References
Park SY, Ahn CB, Je JY (2014) Antioxidant and anti-inflammatory activities of protein hydrolysates from Mytilus edulis and ultrafiltration membrane fractions. J Food Biochem 38:460–468
Chakrabarti S, Wu J (2016) Bioactive peptides on endothelial function. Food Sci Hum Wellness 5:1–7
Hartmann R, Meisel H (2007) Food-derived peptides with biological activity: from research to food applications. Curr Opin Biotechnol 18:163–169
Athmani N, Dehiba F, Allaoui A, Barkia A, Bougatef A, Lamri-Senhadji MY, Moncef N, Boualga A (2015) Sardina pilchardus and Sardinella aurita protein hydrolysates reduce cholesterolemia and oxidative stress in rat fed high cholesterol diet. J Exp Integr Med 5:47–54
Ferraro V, Carvalho AP, Piccirillo C, Santos MM, Castro PM, Pintado ME (2013) Extraction of high added value biological compounds from sardine, sardine-type fish and mackerel canning residues—a review. Mater Sci Eng C Mater Biol Appl 33:3111–3120
Vieira EF, Ferreira IMPLVO (2016) Antioxidant and antihypertensive hydrolysates obtained from by-products of cannery sardine and brewing industries. Int J Food 20:662–673
Williams R, Dias DA, Jayasinghe N, Roessner U, Bennett LE (2016) Beta-glucan-depleted, glycopeptide-rich extracts from Brewer’s and Baker’s yeast (Saccharomyces cerevisiae) lower interferon-gamma production by stimulated human blood cells in vitro. Food Chem 197:761–768
Vieira EF, Carvalho J, Pinto E, Cunha S, Almeida AA, Ferreira IMPLVO (2016) Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J Food Compost Anal 52:44–51
Vieira EF, das Neves J, Vitorino R, Dias da Silva D, Carmo H, Ferreira IMPLVO (2016) Impact of in vitro gastrointestinal digestion and transepithelial transport on antioxidant and ACE-inhibitory activities of brewer’s spent yeast autolysate. J Agric Food Chem 64:7335–7341
Amorim M, Pereira J, Monteiro K, Ruiz A, Pinheiro H, Carvalho J, Pintado M (2014) Antiulcerative and antitumoral properties of spent brewer’s yeast peptide extracts for incorporation in foods. Book of Abstracts of the 1st Congress on Food Structure Design. Braga: Universidade do Minho. ISBN 978-989-97478-5-2. p 85
Hwang JW, Lee SJ, Kim YS, Kim EK, Ahn CB, Jeon YJ, Moone S-H, Jeone B-T, Park PJ (2012) Purification and characterization of a novel peptide with inhibitory effects on colitis induced mice by dextran sulfate sodium from enzymatic hydrolysates of Crassostrea gigas. Fish Shellfish Immunol 33:993–999
Lee S-J, Kim E-K, Kim Y-S, Hwang J-W, Lee KH, Choi D-K, Kang H, Moon S-H, Jeon B-T, Park P-J (2012) Purification and characterization of a nitric oxide inhibitory peptide from Ruditapes philippinarum. Food Chem Toxicol 50:1660–1666
Kim YS, Ahn CB, Je JY (2016) Anti-inflammatory action of high molecular weight Mytilus edulis hydrolysates fraction in LPS-induced RAW264.7 macrophage via NF-kappaB and MAPK pathways. Food Chem 202:9–14
Cheng M-L, Wang H-C, Hsu K-C, Hwang J-S (2015) Anti-inflammatory peptides from enzymatic hydrolysates of tuna cooking juice. Food Agric Immunol 26:770–781
Ko S-C, Jeon Y-J (2014) Anti-inflammatory effect of enzymatic hydrolysates from Styela clava flesh tissue in lipopolysaccharide-stimulated RAW 264.7 macrophages and in vivo zebrafish model. Nutr Res Pract 9:219–226
Kangsanant S, Thongraung C, Jansakul C, Murkovic M, Seechamnanturakit V (2015) Purification and characterisation of antioxidant and nitric oxide inhibitory peptides from Tilapia (Oreochromis niloticus) protein hydrolysate. Int J Food Sci Technol 50:660–665
Rho H-S, Kim H, Kim J, Karadeniz F, Ahn B-N, Nam K-H, Youngwan SY, Kong C-S (2015). Anti-inflammatory effect of by-products from Haliotis discus hannai in RAW 264.7 Cells. J Chem 2015:1–7, Article ID 526439. doi:10.1155/2015/526439
Ahn CB, Cho YS, Je JY (2015) Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem 168:151–156
Kim E-K, Kim Y-S, Hwang J-W, Kang SH, Choi D-K, Lee K-H, Lee JS, Moon S-H, Jeon B-T, Park P-J (2013) Purification of a novel nitric oxide inhibitory peptide derived from enzymatic hydrolysates of Mytilus coruscus. Fish Shellfish Immunol 34:1416–1420
Lin MC, Lin SB, Lee SC, Lin CC, Hui CF, Chen JY (2010) Antimicrobial peptide of an anti-lipopolysaccharide factor modulates of the inflammatory response in RAW264.7 cells. Peptides 31:1262–1272
Ahn C-B, Je J-Y, Cho Y-S (2012) Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Res Int 49:92–98
Bjørndal B, Berge C, Ramsvik MS, Svardal A, Bohov P, Skorve J, Berge RK (2013) A fish protein hydrolysate alters fatty acid composition in liver and adipose tissue and increases plasma carnitine levels in a mouse model of chronic inflammation. Lipids Health Dis 12:143–154
Marchbank T, Limdi JK, Mahmood A, Elia G, Playford RJ (2008) Clinical trial: protective effect of a commercial fish protein hydrolysate against indomethacin (NSAID)-induced small intestinal injury. Aliment Pharmacol Ther 28:799–804
Grootaert C, Kamiloglu S, Capanoglu E, Van Camp J (2015) Cell systems to investigate the impact of polyphenols on cardiovascular health. Nutrients 7:9229–9255
Mukhopadhya A, Noronha N, Bahar B, Ryan MT, Murray BA, Kelly PM, O’Loughlin IB, O’Doherty JV, Sweeney T (2015) The anti-inflammatory potential of a moderately hydrolysed casein and its 5 kDa fraction in in vitro and ex vivo models of the gastrointestinal tract. Food Funct 6:612–621
Kuntz S, Asseburg H, Dold S, Römpp A, Fröhling B, Kunz C, Rudloff S (2015) Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct 6:1136–1149
Toaldo IM, Van Camp J, Gonzales GB, Kamiloglu S, Bordignon-Luiz MT, Smagghe G, Raes K, Capanoglu E, Grootaert C (2016) Resveratrol improves TNF-alpha-induced endothelial dysfunction in a coculture model of a Caco-2 with an endothelial cell line. J Nutr Biochem 36:21–30
Kamiloglu S, Grootaert C, Capanoglu E, Ozkan C, Smagghe G, Raes K, Van Camp J (2016) Anti-inflammatory potential of black carrot (Daucus carota L.) polyphenols in a co-culture model of intestinal Caco-2 and endothelial EA.hy926 cells. Mol Nutr Food Res 61(2). doi:10.1002/mnfr.201600455
Loppnow H, Zhang L, Buerke M, Lautenschläger M, Chen L, Frister A, Schlitt A, Luther T, Song N, Hofmann B, Rose-John S, Silber R-E, Müller-Werdan U, Werdan K (2011) Statins potently reduce the cytokine-mediated IL-6 release in SMC/MNC cocultures. J Cell Mol Med 15:994–1004
Bradford protein assay (2011) Bio-protocol Bio101: e45. http://www.bio-protocol.org/e45/. Accessed 16 March 2001
Cupp-Enyard C (2008) Sigma’s non-specific protease activity assay—casein as a substrate. J Vis Exp (19):e899. doi:10.3791/899
Samaranayaka AG, Kitts DD, Li-Chan EC (2010) Antioxidative and angiotensin-I-converting enzyme inhibitory potential of a Pacific Hake (Merluccius productus) fish protein hydrolysate subjected to simulated gastrointestinal digestion and Caco-2 cell permeation. J Agric Food Chem 58:1535–1542
Malmer MF, Schroeder LA (1990) Amino acid analysis by high-performance liquid chromatography with methanesulfonic acid hydrolysis and 9-fluorenylmethylchloroformate derivatization. J Chromatogr A 514:227–239
Heems D, Luck G, Fraudeau C, Verette E (1998) Fully automated precolumn derivatization, on-line dialysis and high-performance liquid chromatographic analysis of amino acids in food, beverages and feedstuff. J Chromatogr A 798:9–17
Huang SL, Jao CL, Ho KP, Hsu KC (2012) Dipeptidyl-peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides 35:114–121
Kobayashi A, Tanaka H, Hamada Y, Ishizaki S, Nagashima Y, Shiomi K (2006) Comparison of allergenicity and allergens between fish white and dark muscles. Allergy 61(3):357–363
Martínez-Maqueda D, Hernández-Ledesma B, Amigo L, Miralles B, Gómez-Ruiz JÁ (2013) Extraction/fractionation techniques for proteins and peptides and protein digestion. In: Toldrá F, Nollet LML (eds) Proteomics in Foods. Springer, New York, pp 21–50
Voirin A, Letavernier JF, Sebille B (1991) Improvement of extraction and concentration of milk peptides with solid-phase cartridges for analysis by high-performance liquid chromatography. J Chromatogr A 553:155–164
Sun X, Chakrabarti S, Fang J, Yin Y, Wu J (2016) Low-molecular-weight fractions of Alcalase hydrolyzed egg ovomucin extract exert anti-inflammatory activity in human dermal fibroblasts through the inhibition of tumor necrosis factor–mediated nuclear factor κB pathway. Nutr Res 36:648–657
Hasegawa S, Ichiyama T, Sonaka I, Ohsaki A, Okada S, Wakiguchi H, Kudo K, Kittaka S, Hara M, Furukawa S (2012) Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. J Clin Exp Immunol 167:269–274
Suleria HAR, Osborne S, Masci P, Gobe G (2015) Marine-based nutraceuticals: an innovative trend in the food and supplement industries. Mar Drugs 13:6336–6351
Maaser C, Schoeppner S, Kucharzik T, Kraft M, Schoenherr E, Domschke W, Luegering N (2001) Colonic epithelial cells induce endothelial cell expression of ICAM-1 and VCAM-1 by a NF-kappaB-dependent mechanism. Clin Exp Immunol 124:208–213
Acknowledgements
This work received financial support from project UID/QUI/50006/2013—POCI/01/0145/FEDER/007265 with financial support from FCT/MEC through national funds and co-financed by FEDER, under the Partnership Agreement PT2020 and from BOF (Special Research Fund of Ghent University) for their financial support (Project 01B04212). One of the authors (Elsa F. Vieira) wishes to thank the Fundação para a Ciência e a Tecnologia, Portugal, the Grant SFRH/BD/81845/2011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Rights and permissions
About this article
Cite this article
Vieira, E.F., Van Camp, J., Ferreira, I.M.P.L.V.O. et al. Protein hydrolysate from canned sardine and brewing by-products improves TNF-α-induced inflammation in an intestinal–endothelial co-culture cell model. Eur J Nutr 57, 2275–2286 (2018). https://doi.org/10.1007/s00394-017-1503-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1503-2