Abstract
Purpose
To determine the effects of a diet containing fish oil (FD) during pregnancy and lactation in rats on the metabolic adaptations made by the offspring during early extrauterine life and to compare it to an olive oil diet (OD).
Methods
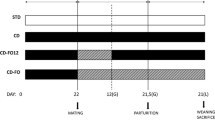
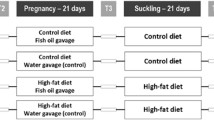
Rats were mated and randomly allocated to OD or FD containing 10 % of the corresponding oil. During lactation, litters were adjusted to eight pups per dam. Fetuses of 20 days and pups of 0, 1, 10, 20 and 30 days of age were studied.
Results
Body weight and length were lower in pups of the FD group from birth. The diet, milk, pups’ plasma and liver of FD group had higher proportions of n-3 LCPUFA, but the content of arachidonic acid (ARA) was lower. Plasma glucose was higher, but unesterified fatty acids, triacylglycerols (TAG), 3-hydroxybutyrate and liver TAG in 1-day-old pups were lower in the FD group, and differences in some of these variables were also found in pups up to 30 days old. Liver lipoprotein lipase activity and mRNA expression, and the expression of carnitine palmitoyl transferase I, acyl-CoA oxidase and 3-hydroxy 3-methyl glutaryl-CoA synthase increased more at birth in pups of the FD group, but the expression of sterol regulatory element binding protein-1c and Δ6-desaturase mRNA was lower in the FD group.
Conclusions
Maternal intake of high n-3 LCPUFA retards postnatal development, which could be the result of impaired ARA synthesis, and affects hepatic metabolic adaptations to extrauterine life.



Similar content being viewed by others
References
Amusquivar E, Rupérez FJ, Barbas C, Herrera E (2000) Low arachidonic acid rather than α-tocopherol is responsible for the delayed postnatal development in offspring of rats fed fish oil instead of olive oil during pregnancy and lactation. J Nutr 130:2855–2865
Amusquivar E, Schiffner S, Herrera E (2011) Evaluation of two methods for plasma fatty acid analysis gy GC. Eur J Lipid Sci Technol 113:711–716
Ballard FJ, Hanson RW (1967) Phosphoenolpyruvate carboxykinase and pyruvate carboxylase in developing rat liver. Biochem J 104:866
Beaudry MA, Chiasson JL, Exton JH (1977) Gluconeogenesis in the suckling rat. Am J Physiol 233:E175–E180
Bordin P, Bodamer OA, Venkatesan S, Gary RM, Bannister PA, Hallyday D (1998) Effects of fish oil supplementation on apolipoprotein B 100 production and lipoprotein metabolism in normolipidaemic males. Eur J Clin Nutr 52:104–109
Botolin D, Jump DB (2003) Selective proteolytic processing of rat hepatic sterol regulatory element binding protein-1 (SREBP-1) and SREBP-2 during postnatal development. J Biol Chem 278:6959–6962
Botolin D, Wang Y, Christian B, Jump DB (2006) Docosahexaenoic acid (22:6, n-3) regulates rat hepatocyte SREBP-1 nuclear abundance by Erk- and 26S proteosome-dependent pathways. J Lipid Res 47:181–192
Christiansen EN, Lund JS, Rortveit T, Rustan AC (1991) Effect of dietary n-3 and n-6 fatty acids on fatty acid desaturation in rat liver. Biochim Biophys Acta 1082:57–62
Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW (1980) Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev 4:121–129
Connor WE, Lowensohn R, Hatcher L (1996) Increased docosahexaenoic acid levels in human newborn infants by administration of sardines and fish oil during pregnancy. Lipids 31:S183–S187
Demmelmair H, Schenck UV, Behrendt E, Sauerwald T, Koletzko B (1995) Estimation of arachidonic acid synthesis in full term neonates using natural variation of 13C-abundance. J Pediatr Gastroenterol Nutr 21:31–36
Ferré P, Decaux JF, Issad T, Girard J (1986) Changes in energy metabolism during the suckling and weaning period in the newborn. Reprod Nutr Dev 26:619–631
Ferré P, Satabin P, El Manoubi L, Callikan S, Grard J (1981) Relationship between ketogenesis and gluconeogenesis in isolated hepatocytes from newborn rats. Biochem J 200:429–433
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 22:24–36
Foreman-van Drongelen MMHP, Van Houwelingen AC, Kester ADM, Hasaart THM, Blanco CE, Hornstra G (1995) Long-chain polyunsaturated fatty acids in preterm infants: status at birth and its influence on postnatal levels. J Pediatr 126:611–618
Girard J, Chatelain F, Boillot J, Prip-Buus C, Thumelin S, Pégorier JP, Foufelle F, Ferré P (1997) Nutrient regulation of gene expression. J Anim Sci 75(suppl. 2):46–57
González MC, Panadero MI, Herrera E, Bocos C (2007) PPARα as target for pharmacological and nutritional agents affecting lipid metabolism. In: Vázquez-Carrera M (ed) New emerging pharmacological targets in metabolic diseases. Transworld Research Network, Kerala, pp 1–48
Grinberg DR, Ramirez I, Vilaró S, Reina M, Llobera M, Herrera E (1985) Starvation enhances lipoprotein lipase activity in the liver of the newborn rat. Biochim Biophys Acta 833:217–222
Gustafsson J (2009) Neoanatl energy substrate production. Indian J Med Res 130:618–623
Haggarty P (2004) Effect of placental function on fatty acid requirements during pregnancy. Eur J Clin Nutr 58:1559–1570
Halminski MA, Marsh JB, Harrison EH (1991) Comparative effects of dietary fish oil (FO), safflower oil (SO) and palm oil (PO) on selected enzymes of hepatic lipid metabolism. In: Simopoulos AP, Kiefer RR, Martin RE, Barlow SM (eds) Textbook of health effects of ω-3 polyunsaturated fatty acids in seafood. Karger, Basel, p 552
Harris WS, Lu G, Rambjor GS, Walen AI, Ontko JA, Cheng QE, Windsor SH (1997) Influence of n-3 fatty acid supplementation on the endogenous activities of plasma lipases. Am J Clin Nutr 66:254–260
Herrera E, Amusquivar E (2000) Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev 16:202–210
Julve J, Robert MQ, Llobera M, Peinado-Onsurbe J (1996) Hormonal regulation of lipoprotein lipase activity from 5-day-old rat hepatocytes. Mol Cell Endocrinol 116:97–104
Jump DB (2004) Fatty acid regulation of gene transcription. Crit Rev Clin Lab Sci 41:41–78
Jump DB, Botolin D, Wang Y (2005) Fatty acid regulation of hepatic gene transcription. J Nutr 135:2503–2506
Jump DB, Oppenheimer JH (1985) High basal expression and 3,5,3ʹ-triiodothyronine regulation of messenger ribonucleic acid S14 in lipogenic tissues. Endocrinology 117:2259–2266
Jumpsen J, Van Aerde J, Clandinin MT (1997) Fetal lipid requirements: implications in fetal growth retardation. In: Battaglia FC (ed) Placental function and fetal nutrition. Nesttec Ltd., Vevey/Lippincott-Raven Publication, Philadelphia, pp 157–165
Kalhan S, Parimi P (2000) Gluconeogenesis in the fetus and neonate. Semin Perinatol 24:94–106
Krauss-Etschmann S, Shadid R, Campoy C, Hoster E, Demmelmair H, Jimenez M, Gil A, Rivero M, Veszpremi B, Decsi T, Koletzko BV (2007) Effects of fish-oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: a European randomized multicenter trial. Am J Clin Nutr 85:1392–1400
Lauritzen L, Jorgensen MH, Mikkelsen TB, Skovgaard IM, Straarup EM, Olsen SF, Hoy CE, Michaelsen KF (2004) Maternal fish oil supplementation in lactation: effect on visual acuity and n-3 fatty acid content of infant erythrocytes. Lipids 39:195–206
Leaf AA, Leightfield MJ, Casteloe KL, Crawford MA (1992) Long-chain polyunsaturated fatty acids and fetal growth. Early Hum Dev 30:183–191
Llobera M, Montes A, Herrera E (1979) Lipoprotein lipase activity in liver of the rat fetus. Biochem Biophys Res Commun 91:272–277
Lockwood EA, Bailey E (1971) The course of ketosis nd the activity of key enzymes of ketogenesis and ketone-body utilization during development of the postnatal rat. Biochem J 124:249–254
Lombardo Y, Chicco A (2006) Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and human. A review. J Nutr Biochem 17:1–13
Lopez Tejero D, Llobera M, Herrera E (1988) High liver lipoprotein lipase activity in hyperlipemic developing rats from undernourished pregnant mothers. Biosci Rep 8:309–314
Matorras R, Perteagudo L, Sanjurjo P, Ruiz JI (1999) Intake of long chain w3 polyunsaturated fatty acids during pregnancy and the influence of levels in the mother on newborn levels. Eur J Obstet Gynecol Reprod Biol 83:179–184
Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Yoshikawa T, Hasly AH, Tamura Y, Osuda J, Okazaki J, Iizuka Y, Takahashi A, Sone H, Gotoda T, Ishibashi S, Yamada N (2002) Dual regulation of mouse Delta(5)- and Delta(6)-desaturase gene expression by SREBP-1 and PPARalpha. J Lipid Res 43:107–141
Neuringer M, Connor WE (1986) Omega-3 fatty acids in the brain and retina: evidence of their essentiality. Nutr Rev 44:285–294
Panadero M, Bocos C, Herrera E (2006) Relationship between lipoprotein lipase and peroxisome proliferator-activated receptor-α expression in rat liver during development. J Physiol Biochem 62:189–198
Panadero M, González MC, Herrera E, Bocos C (2009) Factors modulating fibrates response: therapeutic implications and alternative strategies. Endocr Metab Immune Disord Drug Targets 9:219–239
Park Y, Harris WS (2003) Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J Lipid Res 44:455–463
Perez-Castillo A, Schwartz HL, Oppenheimer JH (1987) Rat hepatic mRNA-S14 and lipogenic enzymes during weaning: role of S14 in lipogenesis. Am J Physiol Endocrinol Metab 253:ES36–ES42
Philippidis H, Ballard FJ (1969) The development of gluconeogenesis in rat liver. Biochem J 113:651–657
Ramirez I, Llobera M, Herrera E (1983) Circulating triacylglycerols, lipoproteins, and tissue lipoprotein lipase activities in rat mothers and offspring during the perinatal period: effect of postmaturity. Metabolism 32:333–341
Raz A, Kamin-Belsky N, Przedecki F, Obukowicz M (1998) Dietary fish oil inhibits Delta6-desaturase activity in vivo. J Am Oil Chem Soc 75:241–245
Raz A, Kamin-Belsky N, Przedecki F, Obukowicz MG (1997) Fish oil inhibits delta 6 desaturase activity in vivo: utility in a dietary paradigm to obtain mice depleted of arachidonic acid. J Nutr Biochem 8:558–565
Reina M, Vilaró S, Ramirez I, Llobera M (1987) Characterization of lipoprotein lipase activity in the newborn rat liver. Biol Neonate 51:45–52
Salem N Jr, Wegher B, Mena P, Uauy R (1996) Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci USA 93:49–54
Sampath H, Ntambi JM (2005) Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu Rev Nutr 25:317–340
Semenkovich CF, Chen S-H, Wims M, Luo C-C, Li W-H, Chan L (1989) Lipoprotein lipase and hepatic lipase mRNA tissue specific expression, developmental regulation, and evolution. J Lipid Res 30:423–431
Somogyi M (1945) Determination of blood sugar. J Biol Chem 160:69–73
Sunehag A, Gustafsson J, Ewald U (1996) Glycerol carbon contributes to hepatic glucose production during the first eight hours in healthy term infants. Acta Paediatr 85:1339–1343
Szitanyi P, Koletzko B, Mydlilova A, Demmelmair H (1999) Metabolism of 13C-labeled linoleic acid in newborn infants during the first week of life. Pediatr Res 45:669–673
Uauy R, Mena P, Wegher B, Nieto S, Salem N Jr (2000) Long chain polyunsaturated fatty acid formation in neonates: effect of gestational age and intrauterine growth. Pediatr Res 47:127–135
Uauy-Dagach R, Mena P (1995) Nutritional role of omega-3 fatty acids during the perinatal period. Clin Perinatol 22:157–175
Van Houwelingen AC, Sorensen JD, Hornstra G, Simonis MMG, Boris J, Olsen SF, Secher NJ (1995) Essential fatty acid status in neonates after fish-oil supplementation during late pregnancy. Br J Nutr 74:723–731
Velzing-Aarts FV, Van der Klis FRM, Van der Dijs FPL, Van Beusekom CM, Landman H, Capello JJ, Muskiet FAJ (2001) Effect of three low-dose fish oil supplements, administered during pregnancy, on neonatal long-chain polyunsaturated fatty acid status at birth. Prostaglandins Leukot Essent Fatty Acids 65:51–57
Vernon RG, Walker DG (1970) Glycerol metabolism in the neonatal rat. Biochem J 118:531–536
Vernon RG, Walker DG (1972) Glucose metabolism in the developing rat. Biochem J 127:521–529
Wang Y, Botolin D, Christian B, Busik J, Xu J, Jump DB (2005) Tissue-specific, nutritional and developmental regulation of rat fatty acid elongases. J Lipid Res 46:706–715
Warstdt K, Furuhjelm C, Duchén K, Falth-Magnusson K, Fageras M (2009) The effects of omega-3 fatty acid supplementation in pregnancy on maternal eicosanoid, cytokine, and chemokine secretion. Pediatr Res 66:212–217
Williamson DH, Mellanby T, Krebs HA (1962) An enzymatic determination of D-β-hydroxybutyric acid and acetonic acid in blood. Biochem J 82:90–96
Willunsen N, Vaagenes H, Lie O, Ustan AC, Berge RG (1996) Eicosapentaenoic acid, but not docosahexaenoic acid, increases mitochondrial fatty acid oxidation and regulates 2,4-dienoyl-CoA reductase gene expression in rats. Lipids 31:579–592
Xu J, Nakamura T, Cho HP, Clarke SD (1999) Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. J Biol Chem 274:23577–23583
Acknowledgments
We thank Milagros Morante for her excellent technical assistance and pp-science-editing.com for editing and linguistic revision of the manuscript.
Author contributions
M.J.J. conducted the research; C.B. conducted the research and analyzed data; M.P. carried out the statistical analysis; E.H. designed research, had primary responsibility for final content and wrote the paper.
Funding
This study was carried out with the financial support of the Universidad San Pablo CEU (USP09-12), the Fundación Ramón Areces of Spain (CIVP 16A1835), the Instituto de Salud Carlos III-Subdirección General de Evaluación y Fomento de la Investigación (PI-09/02192) and the European Community FEDER funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Rights and permissions
About this article
Cite this article
Jiménez, M.J., Bocos, C., Panadero, M. et al. Fish oil diet in pregnancy and lactation reduces pup weight and modifies newborn hepatic metabolic adaptations in rats. Eur J Nutr 56, 409–420 (2017). https://doi.org/10.1007/s00394-015-1091-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1091-y