Abstract
Purpose
To study, in high-fructose-fed rats, the effect of a dietary enrichment in omega-3 polyunsaturated fatty acids (n-3 PUFA) on the expression of genes involved in lipid metabolism and cardiovascular function.
Methods
Twenty-eight male “Wistar Han” rats received for 8 weeks, either a standard chow food or an isocaloric 67 % fructose diet enriched or not in alpha-linolenic acid (ALA) or in docosahexaenoic (DHA) and eicosapentaenoic acids (EPA) mix (DHA/EPA). After sacrifice, blood was withdrawn for biochemical analyses; heart, periepididymal adipose tissue and liver were collected and analyzed for the expression of 22 genes by real-time PCR.
Results
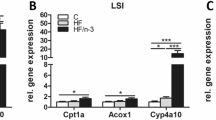
Fructose intake resulted in an increase in liver weight and triglyceride content, plasma triglyceride and cholesterol concentrations, although no difference in glucose and insulin. In the liver, lipogenesis was promoted as illustrated by an increase in stearoyl-CoA desaturase and fatty acid synthase (Fasn) together with a decrease in PPAR gamma, delta and PPAR gamma coactivator 1 alpha (PGC1 alpha) expression. In the heart, Fasn and PPAR delta expression were increased. The addition of ALA or DHA/EPA into the diet resulted in a protection against fructose effects except for the decreased expression of PPARs in the liver that was not counterbalanced by n-3 PUFA suggesting that n-3 PUFA and fructose act independently on the expression of PPARs and PGC1 alpha.
Conclusions
In liver, but not in heart, the fructose-enriched diet induces an early tissue-specific reduction in PPAR gamma and delta expression, which is insensitive to n-3 PUFA intake and dissociated from lipogenesis.



Similar content being viewed by others
References
Bray GA, Nielsen SJ, Popkin BM (2004) Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79:537–543
Miller A, Adeli K (2008) Dietary fructose and the metabolic syndrome. Curr Opin Gastroenterol 24:204–209
Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB (2009) Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 89:1037–1042
Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, Chen G, Fong TH, Lee V, Menorca RI, Keim NL, Havel PJ (2011) Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab 96:E1596–E1605
Tappy L, Le KA (2010) Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90:23–46
Yoshida M, McKeown NM, Rogers G, Meigs JB, Saltzman E, D’Agostino R, Jacques PF (2007) Surrogate markers of insulin resistance are associated with consumption of sugar-sweetened drinks and fruit juice in middle and older-aged adults. J Nutr 137:2121–2127
Havel PJ (2005) Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev 63:133–157
Hwang IS, Ho H, Hoffman BB, Reaven GM (1987) Fructose-induced insulin resistance and hypertension in rats. Hypertension 10:512–516
Kazumi T, Odaka H, Hozumi T, Ishida Y, Amano N, Yoshino G (1997) Effects of dietary fructose or glucose on triglyceride production and lipogenic enzyme activities in the liver of Wistar fatty rats, an animal model of NIDDM. Endocr J 44:239–245
Kok N, Roberfroid M, Delzenne N (1996) Dietary oligofructose modifies the impact of fructose on hepatic triacylglycerol metabolism. Metabolism 45:1547–1550
Panchal SK, Poudyal H, Iyer A, Nazer R, Alam A, Diwan V, Kauter K, Sernia C, Campbell F, Ward L, Gobe G, Fenning A, Brown L (2011) High-carbohydrate high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol 57:51–64
Basciano H, Federico L, Adeli K (2005) Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2:5
Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Tomita S, Sekiya M, Hasty A, Nakagawa Y, Sone H, Toyoshima H, Ishibashi S, Osuga J, Yamada N (2004) Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes 53:560–569
Nagai Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kashiwagi A (2002) Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am J Physiol Endocrinol Metab 282:E1180–E1190
Rutledge AC, Adeli K (2007) Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev 65:S13–S23
Morino K, Petersen KF, Shulman GI (2006) Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55(Suppl 2):S9–S15
Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI (2004) Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279:32345–32353
Stanhope KL, Havel PJ (2008) Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr Opin Lipidol 19:16–24
Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S (2011) Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123:2292–2333
Ayalew-Pervanchon A, Rousseau D, Moreau D, Assayag P, Weill P, Grynberg A (2007) Long-term effect of dietary {alpha}-linolenic acid or decosahexaenoic acid on incorporation of decosahexaenoic acid in membranes and its influence on rat heart in vivo. Am J Physiol Heart Circ Physiol 293:H2296–H2304
Bucher HC, Hengstler P, Schindler C, Meier G (2002) N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med 112:298–304
Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, Cunnane SC, Holden JM, Klurfeld DM, Morris MC, Whelan J (2009) Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr 139:804S–819S
Jump DB, Depner CM, Tripathy S (2012) Omega-3 fatty acid supplementation and cardiovascular disease. J Lipid Res (in press). doi:10.1194/jlr.R027904
Rousseau D, Helies-Toussaint C, Moreau D, Raederstorff D, Grynberg A (2003) Dietary n-3 PUFAs affect the blood pressure rise and cardiac impairments in a hyperinsulinemia rat model in vivo. Am J Physiol Heart Circ Physiol 285:H1294–H1302
Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L (2005) Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 54:1907–1913
Jump DB, Clarke SD (1999) Regulation of gene expression by dietary fat. Annu Rev Nutr 19:63–90
Lang CA, Davis RA (1990) Fish oil fatty acids impair VLDL assembly and/or secretion by cultured rat hepatocytes. J Lipid Res 31:2079–2086
Clarke SD, Jump D (1997) Polyunsaturated fatty acids regulate lipogenic and peroxisomal gene expression by independent mechanisms. Prostaglandins Leukot Essent Fatty Acids 57:65–69
Wendland E, Farmer A, Glasziou P, Neil A (2006) Effect of alpha linolenic acid on cardiovascular risk markers: a systematic review. Heart 92:166–169
Robbez Masson V, Lucas A, Gueugneau AM, Macaire JP, Paul JL, Grynberg A, Rousseau D (2008) Long-chain (n-3) polyunsaturated fatty acids prevent metabolic and vascular disorders in fructose-fed rats. J Nutr 138:1915–1922
Truong H, DiBello JR, Ruiz-Narvaez E, Kraft P, Campos H, Baylin A (2009) Does genetic variation in the Delta6-desaturase promoter modify the association between alpha-linolenic acid and the prevalence of metabolic syndrome? Am J Clin Nutr 89:920–925
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Catena C, Giacchetti G, Novello M, Colussi G, Cavarape A, Sechi LA (2003) Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. Am J Hypertens 16:973–978
Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B (2006) Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17:4–12
Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S (2009) Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 106:15430–15435
Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H (2002) Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 87:3023–3028
Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, Ntambi JM (2002) Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res 43:1899–1907
Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM (2000) The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem 275:30132–30138
Akiyama TE, Lambert G, Nicol CJ, Matsusue K, Peters JM, Brewer HB Jr, Gonzalez FJ (2004) Peroxisome proliferator-activated receptor beta/delta regulates very low density lipoprotein production and catabolism in mice on a Western diet. J Biol Chem 279:20874–20881
Barroso E, Rodriguez-Calvo R, Serrano-Marco L, Astudillo AM, Balsinde J, Palomer X, Vazquez-Carrera M (2011) The PPARbeta/delta activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1alpha-Lipin 1-PPARalpha pathway leading to increased fatty acid oxidation. Endocrinology 152:1848–1859
Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W (1996) Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 137:354–366
Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q (2004) Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med 10:1245–1250
Barish GD, Narkar VA, Evans RM (2006) PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest 116:590–597
Ntambi JM (1999) Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res 40:1549–1558
Rodriguez-Cruz M, Sanchez Gonzalez R, Sanchez Garcia AM, Lopez-Alarcon M (2012) Coexisting role of fasting or feeding and dietary lipids in the control of gene expression of enzymes involved in the synthesis of saturated, monounsaturated and polyunsaturated fatty acids. Gene 496:28–36
Qin X, Xie X, Fan Y, Tian J, Guan Y, Wang X, Zhu Y, Wang N (2008) Peroxisome proliferator-activated receptor-delta induces insulin-induced gene-1 and suppresses hepatic lipogenesis in obese diabetic mice. Hepatology 48:432–441
Clarke SD, Turini M, Jump DB, Abraham S, Reedy M (1998) Polyunsaturated fatty acid inhibition of fatty acid synthase transcription is independent of PPAR activation. Z Ernahrungswiss 37(Suppl 1):14–20
Taouis M, Dagou C, Ster C, Durand G, Pinault M, Delarue J (2002) N-3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol Endocrinol Metab 282:E664–E671
Bray GA (2012) Fructose and risk of cardiometabolic disease. Curr Atheroscler Rep (in press). doi:10.1007/s11883-012-0276-6
Acknowledgments
The authors thank Pr Michel Miniconi for the supervision of statistical tests. This work was supported by the European LipGene project (EU sixth Framework Integrated Program, contract FOOD-CT-2003-505944) WP 1.3.5.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karsenty, J., Landrier, JF., Rousseau-Ralliard, D. et al. Beneficial effects of omega-3 fatty acids on the consequences of a fructose diet are not mediated by PPAR delta or PGC1 alpha. Eur J Nutr 52, 1865–1874 (2013). https://doi.org/10.1007/s00394-012-0488-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0488-0