Abstract
Aim of study
The purpose of the study was to evaluate hypolipidemic and hypocholesterolemic activities of conjugated linolenic acid (CLnA) isomers, present in bitter gourd and snake gourd seed, in terms of amelioration of plasma lipid profile, lipoprotein oxidation and erythrocyte membrane fluidity after oral administration.
Methods
Male albino rats were divided into six groups. Group 1 was control, and others were induced with oxidative stress by oral gavage of sodium arsenite (Sa). Group 2 was kept as treated control, and groups 3–6 were further treated with different oral doses of seed oils to maintaining definite concentration of CLnA isomers (0.5 and 1.0% of total lipid for each CLnA isomer).
Results
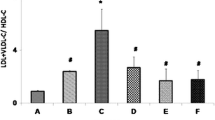
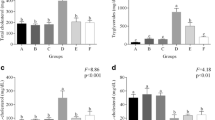
CLnA isomers normalized cholesterol, LDL-cholesterol, HDL-cholesterol and triglyceride contents in plasma and body weight of experimental rats and decreased cholesterol synthesis by reducing hepatic HMG-CoA reductase activity. Administration of Sa caused alteration in erythrocyte membrane fluidity due to increase in cholesterol and decrease in phospholipid content. Tissue cholesterol and lipid contents were also increased by Sa administration. These altered parameters were reversed by experimental oil administration. Protective effect of CLnA isomers on erythrocyte morphology was observed by atomic force microscopy (AFM). Fatty acid composition of erythrocyte membrane showed decrease in polyunsaturated fatty acid (PUFA) and increase in arachidonic acid content after Sa administration, which was normalized with the treatment of these oils. Supplementation of CLnA isomers restored erythrocyte membrane (EM) lipid peroxidation and lipoprotein oxidation.
Conclusion
CLnA isomers, present in vegetable oils, showed potent hypolipidemic and hypocholesterolemic activities against biochemical perturbations.







Similar content being viewed by others
References
Mukherjee C, Bhattacharyya S, Ghosh S, Bhattacharyya DK (2002) Dietary effects of punicic acid on the composition and peroxidation of rat plasma lipid. J Oleo Sci 51:513–522
Fernandez ML, West KL (2005) Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr 135:2075–2078. doi:135/9/2075[pii]
Dhar P, Ghosh S, Bhattacharyya DK (1999) Dietary effects of conjugated octadecatrienoic fatty acid (9 cis, 11 trans, 13 trans) levels on blood lipids and nonenzymatic in vitro lipid peroxidation in rats. Lipids 34:109–114
Kim BJ, Kim YK, Park WH, Ko JH, Lee YC, Kim CH (2003) A water-extract of the Korean traditional formulation Geiji-Bokryung-Hwan reduces atherosclerosis and hypercholesteremia in cholesterol-fed rabbits. Int Immunopharmacol 3:723–734. doi:S1567-5769(03)00073-0[pii]10.1016/S1567-5769(03)00073-0
Rosenson RS (2004) Statins in atherosclerosis: lipid-lowering agents with antioxidant capabilities. Atherosclerosis 173:1–12. doi:10.1016/S0021-9150(03)00239-9S0021915003002399[pii]
Hegsted DM, McGandy RB, Myers ML, Stare FJ (1965) Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr 17:281–295
Takada R, Saitoh M, Mori T (1994) Dietary gamma-linolenic acid-enriched oil reduces body fat content and induces liver enzyme activities relating to fatty acid beta-oxidation in rats. J Nutr 124:469–474
Gerasimova E, Perova N, Ozerova I, Polessky V, Metelskaya V, Sherbakova I, Levachev M, Kulakova S, Nikitin Y, Astakhova T (1991) The effect of dietary n-3 polyunsaturated fatty acids on HDL cholesterol in Chukot residents vs muscovites. Lipids 26:261–265
Fukushima M, Akiba S, Nakano M (1996) Comparative hypocholesterolemic effects of six vegetable oils in cholesterol-fed rat. Lipids 31:415–419
Clemens MR, Ruess M, Bursa Z, Waller HD (1987) The relationship between lipid composition of red blood cells and their susceptibility to lipid peroxidation. Free Radic Res Commun 3:265–271
Scott MD, van den Berg JJ, Repka T, Rouyer-Fessard P, Hebbel RP, Beuzard Y, Lubin BH (1993) Effect of excess alpha-hemoglobin chains on cellular and membrane oxidation in model beta-thalassemic erythrocytes. J Clin Invest 91:1706–1712. doi:10.1172/JCI116380
Berlin E, Bhathena SJ, McClure D, Peters RC (1998) Dietary menhaden and corn oils and the red blood cell membrane lipid composition and fluidity in hyper- and normocholesterolemic miniature swine. J Nutr 128:1421–1428
Vidgren HM, Agren JJ, Schwab U, Rissanen T, Hanninen O, Uusitupa MI (1997) Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil among healthy young men. Lipids 32:697–705
Saha SS, Ghosh M (2009) Comparative study of antioxidant activity of alpha-eleostearic acid and punicic acid against oxidative stress generated by sodium arsenite. Food Chem Toxicol 47:2551–2556. doi:S0278-6915(09)00342-1[pii]10.1016/j.fct.2009.07.012
Saha SS, Ghosh M (2010) Ameliorative role of conjugated linolenic acid isomers against oxidative DNA damage induced by sodium arsenite in rat model. Food Chem Toxicol 48:3398–3405. doi:S0278-6915(10)00574-0[pii]10.1016/j.fct.2010.09.011
Dastgiri S, Mosaferi M, Fizi MA, Olfati N, Zolali S, Pouladi N, Azarfam P (2010) Arsenic exposure, dermatological lesions, hypertension, and chromosomal abnormalities among people in a rural community of northwest Iran. J Health Popul Nutr 28:14–22
Kadirvel R, Sundaram K, Mani S, Samuel S, Elango N, Panneerselvam C (2007) Supplementation of ascorbic acid and alpha-tocopherol prevents arsenic-induced protein oxidation and DNA damage induced by arsenic in rats. Hum Exp Toxicol 26:939–946. doi:26/12/939[pii]10.1177/0960327107087909
Rodriguez VM, Carrizales L, Jimenez-Capdeville ME, Dufour L, Giordano M (2001) The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res Bull 55:301–308. doi:S0361-9230(01)00477-4[pii]
Flora SJ (1999) Arsenic-induced oxidative stress and its reversibility following combined administration of N-acetylcysteine and meso 2, 3-dimercaptosuccinic acid in rats. Clin Exp Pharmacol Physiol 26:865–869
Bhattacharyya AC, Majumdar S, Bhattacharyya DK (1986) Edible quality rice bran oil from high FFA rice bran oil by miscella refining. J Am Oil Chem Soc 63:1189–1191
Ichihara K, Shibahara A, Yamamoto K, Nakayama T (1996) An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids 31:535–539
AOCS (1996) Official methods and recommended practices of the American oil chemists’ society. American Oil Chemists’ Society, Champaign
Jones JH, Foster CA (1942) A salt mixture for use with basal diet either low or high in phosphorus. J Nutr 24:245–256
Rose HG, Oklander M (1965) Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res 6:428–431
Mezick JA, Settlemire CT, Brierley GP, Barefield KP, Jensen WN, Cornwell DG (1970) Erythrocyte membrane interactions with menadione and the mechanism of menadione-induced hemolysis. Biochim Biophys Acta 219:361–371
Brownlee NR, Huttner JJ, Panganamala RV, Cornwell DG (1977) Role of vitamin E in glutathione-induced oxidant stress: methemoglobin, lipid peroxidation, and hemolysis. J Lipid Res 18:635–644
Allain CC, Poon LS, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475
Bucolo G, David H (1973) Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 19:476–482
Warnick GR, Nguyen T, Albers AA (1985) Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin Chem 31:217–222
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Chen PS, Toribara TY, Warner H (1956) Microdetermination of phosphorous. Anal Chem 28:1756–1758
Rao AV, Ramakrishnan S (1975) Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin Chem 21:1523–1525
Bachorik PS, Albers JJ (1986) Precipitation methods for quantification of lipoproteins. Methods Enzymol 129:78–100
Phelps S, Harris WS (1993) Garlic supplementation and lipoprotein oxidation susceptibility. Lipids 28:475–477
Kates M (1972) Techniques of lipidology. American Elsevier Publishing Co., New York
Dani V, Dhawan DK (2005) Radioprotective role of zinc following single dose radioiodine (131I) exposure to red blood cells of rats. Indian J Med Res 122:338–342
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Scheffe H (1961) The analysis of variance.Wiley, New York, 27–28; 73–74
Nicolosi RJ, Rogers EJ, Kritchevsky D, Scimeca JA, Huth PJ (1997) Dietary conjugated linoleic acid reduces plasma lipoproteins and early aortic atherosclerosis in hypercholesterolemic hamsters. Artery 22:266–277
Lee KN, Kritchevsky D, Pariza MW (1994) Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 108:19–25
Noguchi R, Yasui Y, Suzuki R, Hosokawa M, Fukunaga K, Miyashita K (2001) Dietary effects of bitter gourd oil on blood and liver lipids of rats. Arch Biochem Biophys 396:207–212. doi:10.1006/abbi.2001.2624S0003-9861(01)92624-4[pii]
Penn MS, Chisolm GM (1994) Oxidized lipoproteins, altered cell function and atherosclerosis. Atherosclerosis 108(Suppl):S21–S29
Chiu D, Kuypers F, Lubin B (1989) Lipid peroxidation in human red cells. Semin Hematol 26:257–276
Hebbel RP, Leung A, Mohandas N (1990) Oxidation-induced changes in microrheologic properties of the red blood cell membrane. Blood 76:1015–1020
Ip C, Chin SF, Scimeca JA, Pariza MW (1991) Mammary cancer prevention by conjugated dienoic derivative of linoleic acid. Cancer Res 51:6118–6124
Biswas D, Banerjee M, Sen G, Das JK, Banerjee A, Sau TJ, Pandit S, Giri AK, Biswas T (2008) Mechanism of erythrocyte death in human population exposed to arsenic through drinking water. Toxicol Appl Pharmacol 230:57–66. doi:S0041-008X(08)00071-9[pii]10.1016/j.taap.2008.02.003
Evans EA, Hochmuth RM (1977) A solid-liquid composite model of the red cell membrane. J Membr Biol 30:351–362
Chen H, Li S, Liu J, Diwan BA, Barrett JC, Waalkes MP (2004) Chronic inorganic arsenic exposure induces hepatic global and individual gene hypomethylation: implications for arsenic hepatocarcinogenesis. Carcinogenesis 25:1779–1786. doi:10.1093/carcin/bgh161bgh161[pii]
Mann KK, Padovani AM, Guo Q, Colosimo AL, Lee HY, Kurie JM, Miller WH Jr (2005) Arsenic trioxide inhibits nuclear receptor function via SEK1/JNK-mediated RXRalpha phosphorylation. J Clin Invest 115:2924–2933. doi:10.1172/JCI23628
Santra A, Chowdhury A, Ghatak S, Biswas A, Dhali GK (2007) Arsenic induces apoptosis in mouse liver is mitochondria dependent and is abrogated by N-acetylcysteine. Toxicol Appl Pharm 220:146–155. doi:10.1016/j.taap.2006.12.029
Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW (1997) Effect of conjugated linoleic acid on body composition in mice. Lipids 32:853–858
Kim HK, Choi S, Choi H (2004) Suppression of hepatic fatty acid synthase by feeding alpha-linolenic acid rich perilla oil lowers plasma triacylglycerol level in rats. J Nutr Biochem 15:485–492. doi:10.1016/j.jnutbio.2004.02.010S0955286304000579[pii]
Acknowledgments
The work was funded by University of Calcutta under ‘University with Potential for Excellence’ scheme supported by Govt. of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saha, S.S., Chakraborty, A., Ghosh, S. et al. Comparative study of hypocholesterolemic and hypolipidemic effects of conjugated linolenic acid isomers against induced biochemical perturbations and aberration in erythrocyte membrane fluidity. Eur J Nutr 51, 483–495 (2012). https://doi.org/10.1007/s00394-011-0233-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-011-0233-0