Abstract
Background
In human beings, women are at lower risk of cardiovascular diseases, and respond differently from men to dietary fatty acids.
Aim
The aim of the present study was to investigate (i) the influence of gender on the response of lipid metabolism to dietary n-3 PUFA, and (ii) the contribution of PPARα to this response.
Methods
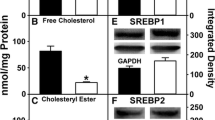
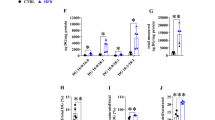
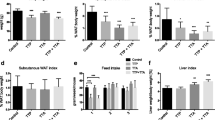
Male and female mice, wild-type (WT) and PPARα-null (KO), were fed on diets rich in either saturated FA (SFA) or 18:3 n-3 (ALA). Lipid composition, mRNA levels and certain activities of key enzymes and major transcription factors were determined in the liver. WT mice were slightly affected by dietary FA. However, in WT female mice, but not in males, mRNA levels of PPARα-dependent genes (L-FABP, ACO) were higher in the mice fed on the ALA-rich diet. When compared to WT mice, KO female mice exhibited a decreased lipogenesis capacity (40% lower FAS, ACC, and SREBP-1c mRNA level), whereas KO males showed a decrease in peroxisomal β-oxidation (activity and expression of ACO reduced by 20 and 40%, respectively). When compared to SFA-fed KO mice, steatosis was twice lower in KO mice fed on ALA, despite the absence of dietary effect on plasma TG, CPT1 and ACO activities, or ACC and FAS expression. Besides, in mice on the SFA diet, steatosis was alleviated in females, and CPT1 expression was up-regulated to a higher extent in females than in males (2.7- and 3.6-fold, respectively, as compared to the corresponding WT groups).
Conclusions
Our data suggests estrogen to modulate the regulation of hepatic lipid metabolic pathway by dietary fatty acids. Besides, PPARα invalidation resulted in unexpected regulations by ALA of its known targets and was compensated partly in females, which was therefore less sensitive to the detrimental effects of a SFA-rich diet.


Similar content being viewed by others
References
Besnard P, Mallordy A, Carlier H (1993) Transcriptional induction of the fatty acid binding protein gene in mouse liver by bezafibrate. FEBS Lett 327:219–223
Bieber LL, Abraham T, Helmrath T (1972) A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal Biochem 50:509–518
Calder PC (2004) n-3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond) 107:1–11
Chomczynski P, Qasba P, Topper YJ (1986) Transcriptional and post-transcriptional roles of glucocorticoid in the expression of the rat 25,000 molecular weight casein gene. Biochem Biophys Res Commun 134:812–818
Cobb M, Greenspan J, Timmons M, Teitelbaum H (1993) Gender differences in lipoprotein responses to diet. Ann Nutr Metab 37:225–236
Costet P, Legendre C, More J, Edgar A, Galtier P, Pineau T (1998) Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol Chem 273:29577–29585
Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP (1998) A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor alpha-deficient mice. J Clin Invest 102:1083–1091
Fossati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28:2077–2080
Isensee J, Ruiz Noppinger P (2007) Sexually dimorphic gene expression in mammalian somatic tissue. Gend Med 4(Suppl B):S75–S95
Kim HJ, Takahashi M, Ezaki O (1999) Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. A possible mechanism for down-regulation of lipogenic enzyme mRNAs. J Biol Chem 274:25892–25898
Lazarow PB, De Duve C (1976) A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci USA 73:2043–2046
Li Z, Otvos JD, Lamon-Fava S, Carrasco WV, Lichtenstein AH, McNamara JR, Ordovas JM, Schaefer EJ (2003) Men and women differ in lipoprotein response to dietary saturated fat and cholesterol restriction. J Nutr 133:3428–3433
Linden D, Alsterholm M, Wennbo H, Oscarsson J (2001) PPAR{alpha} deficiency increases secretion and serum levels of apolipoprotein B-containing lipoproteins. J Lipid Res 42:1831–1840
Loison C, Mendy F, Serougne C, Lutton C (2002) Dietary myristic acid modifies the HDL-cholesterol concentration and liver scavenger receptor BI expression in the hamster. Br J Nutr 87:199–210
Luz Fernandez M, West KL, Roy S, Ramjiganesh T (2001) Dietary fat saturation and gender/hormonal status modulate plasma lipids and lipoprotein composition. J Nutr Biochem 12:703–710
Martin PGP, Guillou H, Lasserre F, Déjean S, Lan A, Pascussi JM, SanCristobal M, Legrand P, Besse P, Pineau T (2007) Novel aspects of PPARα mediated regulation of lipid and xenobiotic metabolism revealed through a nutrigenomic study. Hepatology 45:767–777
Morise A, Combe N, Boue C, Legrand P, Catheline D, Delplanque B, Fenart E, Weill P, Hermier D (2004) Dose effect of alpha-linolenic acid on PUFA conversion, bioavailability, and storage in the hamster. Lipids 39:325–334
Morise A, Serougne C, Gripois D, Blouquit M-F, Lutton C, Hermier D (2004) Effects of dietary alpha linolenic acid on cholesterol metabolism in male and female hamsters of the LPN strain. J Nutr Biochem 15:51–61
Morise A, Mourot J, Boue C, Combe N, Amsler G, Gripois D, Quignard-Boulange A, Yvan-Charvet L, Fenart E, Weill P, Hermier D (2006) Gender-related response of lipid metabolism to dietary fatty acids in the hamster. Br J Nutr 95:709–720
Nunez SB, Medin JA, Braissant O, Kemp L, Wahli W, Ozato K, Segars JH (1997) Retinoid X receptor and peroxisome proliferator-activated receptor activate an estrogen responsive gene independent of the estrogen receptor. Mol Cell Endocrinol 127:27–40
Richmond W (1973) Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem 19:1350–1356
Roeters van Lennep JE, Westerveld HT, Erkelens DW, van der Wall EE (2002) Risk factors for coronary heart disease: implications of gender. Cardiovasc Res 53:538–549
Souidi M, Parquet M, Ferezou J, Lutton C (1999) Modulation of cholesterol 7alpha-hydroxylase and sterol 27-hydroxylase activities by steroids and physiological conditions in hamster. Life Sci 64:1585–1593
Wang X, Kilgore MW (2002) Signal cross-talk between estrogen receptor alpha and beta and the peroxisome proliferator-activated receptor gamma1 in MDA-MB-231 and MCF-7 breast cancer cells. Mol Cell Endocrinol 194:123–133
Wilson TA, Nicolosi RJ, Lawton CW, Babiak J (1999) Gender differences in response to a hypercholesterolemic diet in hamsters: effects on plasma lipoprotein cholesterol concentrations and early aortic atherosclerosis. Atherosclerosis 146:83–91
Acknowledgments
The authors gratefully acknowledge the financial support of ONIDOL (Organisation Nationale Interprofessionnelle des Graines et Fruits Oléagineux) and ONIOL (Office National Interprofessionnel des Oléagineux, protéagineux et cultures textiles). They also thank sincerely Nicole Combe and Carole Boué (ITERG, Talence, France) for dietary fatty acid analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morise, A., Thomas, C., Landrier, JF. et al. Hepatic lipid metabolism response to dietary fatty acids is differently modulated by PPARα in male and female mice. Eur J Nutr 48, 465–473 (2009). https://doi.org/10.1007/s00394-009-0037-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-009-0037-7